- Academic Editor
Gallstone formation is a common digestive ailment, with unclear mechanisms underlying its development. Dysfunction of the gallbladder smooth muscle (GSM) may play a crucial role, particularly with a high-fat diet (HFD). This study aimed to investigate the effects of an HFD on GSM and assess how it alters contractility through changes in the extracellular matrix (ECM).
Guinea pigs and C57BL/6 mice were fed either an HFD or normal diet (ND). Primary cultures of their (guinea pigs) gallbladder smooth muscle cells (GSMCs) were used for in vitro experiments. Histological stains, RNA-sequencing, bioinformatics analysis, three-dimensional tissue culture, real-time polymerase chain reaction (PCR), Western blot, atomic force microscopy, and muscle tension measurements were performed.
Histological evidence indicated structural changes in the gallbladder muscle layer and ECM collagen deposition in the HFD group. The HFD group also showed increased expression of collagen, integrin family, and matrix metalloproteinase (MMP) and the phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB/Akt) signaling pathway. Compared with GSMCs cultured on Matrigel containing 1 mg/mL of collagen I, those cultured with 2 mg/mL showed a phenotype change from contractile to synthetic cells. Consistent with these findings, the HFD group also demonstrated increased ECM stiffness and decreased smooth muscle contractility.
Our findings reveal a mechanism by which an HFD alters the ECM composition of the gallbladder muscle, activating the integrin/PI3K-Akt/MMP signaling pathway, thereby impacting GSMC phenotype and contractility. These insights enhance the understanding of gallstone formation mechanism and provide potential therapeutic targets to treat gallbladder dysfunction.
Gallbladder disease is a common and frequent disorder of the digestive system. The incidence of gallstone formation is 10% to 20% in adults and may lead to serious complications, such as acute suppurative cholangitis, biliary pancreatitis, and gallbladder cancer [1, 2, 3]. The main types of gallstones include cholesterol stones, bile pigment stones, and mixed stones, with cholesterol stones being the most common [4]. The causes of gallstones are associated with bile cholesterol supersaturation, rapid phase transitions of cholesterol crystals, and gallbladder dyskinesia [5, 6]. The existing studies in this field have mainly focused on the rapid phase transition of bile cholesterol supersaturation and cholesterol crystals, with relatively few studies investigating the contribution of gallbladder smooth muscle (GSM) to gallstone formation [7, 8]. GSM contractile dysfunction occurs in the early stages of cholesterol stone formation [9]. Dysfunction of gallbladder contractility plays a key role in stone formation [10]. Thus, it is important to understand dysfunction in GSM contraction. Gallbladder contractile dysfunction is an early precursor to gallstone disease and is considered to be a trigger for gallstone formation [11]. Gallbladder contraction dysfunction will increase the transport time of supersaturated bile, prompting cholesterol crystals to continue to grow. High fat diet (HFD) can lead to lipid deposition in the gallbladder wall, resulting in gallbladder contraction dysfunction [12]. In patients with gallstone disease and animal models, dysfunction of gallbladder contraction can be detected. Another important event associated with gallbladder contractile dysfunction is lipid deposition in the gallbladder wall [13]. In fact, some diseases characterized by ectopic lipid deposition, such as type 2 diabetes, hyperlipidemia, and insulin resistance, are associated with gallbladder contraction dysfunction. Therefore, normal gallbladder structure is essential for maintaining gallbladder function.
The structure and molecular composition of the extracellular matrix (ECM) vary
depending on the location, structure, and function of the organ source. The
gallbladder is a thin-walled organ, and its muscular layer is composed of loose
smooth muscle and rich collagen [14]. Gallbladder contractile dysfunction plays
an important role in the pathogenesis of benign gallbladder diseases and is one of the mail factors in the occurrence of gallstones. The decrease of gallbladder
contractility is result from the increase of fibrosis of gallbladder muscle
layer. Köninger et al. [15] found that Transforming Growth Factor-beta1 (TGF-
The ECM, a fluid three-dimensional network structure, plays a vital role in
maintaining the homeostasis of tissue structure and function. The ECM is composed
mainly of collagen, proteoglycans, glycoproteins, and laminin [17]. Collagen is
the most important component of the ECM, as it provides the structural strength
[18]. ECM composition change is a dynamic equilibrium process, and disturbances
in this balance may lead to disease [19]. Cells sense changes in the
microenvironment in which they live and make changes accordingly. Changes in ECM
properties, such as stiffness, density, and viscosity, are sensed by cells and
result in alterations to cell phenotype and function [20, 21]. For example, airway
smooth muscle cells cultured in a dish containing laminin express increased
In this study, we used in vivo and in vitro experiments to investigate the effects of a HFD on the phenotype and function of the GSM through the ECM to gain new insights on the causes of gallstone formation.
Carbachol (CCh, HY-B1208) and
Male white guinea pigs (250–300 g) and 5-week-old male C57BL/6 mice were
purchased from Hangzhou Yuanyuan Experimental Animal Technology Co., Ltd. and kept
in a constant temperature (25
Guinea pigs were killed by carbon dioxide (CO2) gas asphyxiation, and the
gallbladder was quickly removed under sterile conditions. The gallbladder was
rinsed several times in PBS containing 2% penicillin-streptomycin and quickly
transferred to an ultra-clean workbench. The mucosal and serous layers of the
gallbladder were removed, and then the gallbladder tissue was cut into 1
Three-dimensional cell culture was performed as the description of previous study similarly [32]. Firstly, Matrigel containing 1 mg/mL or 2 mg/mL collagen I was prepared in a centrifuge tube on ice and then transfered to a six-well plates which subsequently placed in an incubator containing 5% CO2 at 37 °C until the Matrigel solidified. Secondly, the above GSMCs were digested with 0.25% trypsin-EDTA and then counted. The number of cells was 10-6. Finally, the sufficient cells were resuspended in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin solution and then mixed with the solid Matrigel at a volume ratio of 1:1. The co-culture system was placed in a humidified atmosphere containing 5% CO2 at 37 °C. The cells were cultured for 48 h and then collected for realTime quantitative polymerase chain reaction (RT-PCR) and Western blot.
Fresh gallbladder tissue (C57BL/6 mice) was fixed in 4% polyoxymethylene and then subjected to gradient dehydration and paraffin embedding prior to sectioning, dewaxing, and washing. The sections were treated with high-definition constant staining pretreatment solution for 1 min. The sections were incubated with hematoxylin stain and then washed, treated with differentiation solution, and washed again. Treat the sections with hematoxylin bluing solution, washed with water. The sections were dehydrated with 95% alcohol and then counterstained with eosin staining solution. The sections were then dehydrated in alcohol, cleared with xylene, coverslipped with mounting medium, and examined under a light microscope. Images were collected and analyzed.
For Masson staining, after the tissue sections were dewaxed and washed, they were soaked overnight in Masson A solution and rinsed with running water. The slices were then soaked in a solution that was equal parts Masson B solution and Masson C solution prior to being washed with water, differentiated with differentiation solution, and washed with water. The sections were impregnated with Masson D solution and washed with water before being impregnated with Masson E solution, lightly drained, and incubated with Masson F solution. Sections were differentiated by rinsing with 1% acetic acid and dehydrated with absolute ethanol prior to being coverslipped. The processed tissue underwent microscopic examination, image acquisition, and analysis.
For EVG staining of elastic fibers, another group of tissue sections was stained with EVG dye solution and washed with water. The degree of differentiation with ferric chloride solution of the elastic fibers was purple-black with an off-white background that was nearly colorless. Sections were counterstained with VG stain, washed with water, dehydrated, cleared with xylene, and coverslipped prior to microscopic examination and image acquisition and analysis.
The RNA from each sample, which consisted of three gallbladder tissue sections per group, was isolated using the TRIzol® reagent from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA) by following the manufacturer’s instructions. The isolated RNA was then processed with RNase-free DNase I for 30 min. Poly(A)-containing mRNA was purified from 1–2 µg of total RNA using Oligo(dT) beads (Beyotime, Shanghai, China). The captured mRNA was fragmented into lengths of between 150 and 300 nucleotides by using divalent cations at a high temperature. The fragmented mRNA underwent reverse transcription using SuperScript™ II (Thermo Fisher Scientific, Waltham, MA, USA) and was subsequently converted into double-stranded cDNA through random priming with the aid of RNase H and DNA polymerase I (Thermo Fisher Scientific, Waltham, MA, USA). After being purified, the double-stranded complementary DNA (cDNA) underwent blunt-end ligation, with the addition of a single adenosine (dA) to the 3′-end and adapter ligation. The final step involved performing PCR to enrich the adapter-ligated cDNA. The libraries were analyzed using an Agilent Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, CA, USA) and quantified via Quantitative Polymerase Chain Reaction (qPCR) before being sequenced on an Illumina sequencing platform (Illumina, San Diego, CA, USA).
A comprehensive dataset comprising 17,632 genes associated with ECM was
retrieved from the GeneCards database (https://www.genecards.org/) [33]. From
this dataset, 210 genes exhibiting correlation scores greater than 16.0 were
selected for subsequent bioinformatics analysis (Supplementary Table 1). The RNA-seq sequencing data of
mice gallbladder tissues were log2 converted for further analysis. The ‘limma’
package was used to recognize differentially expressed genes (DEGs) on the
SangerBox website (www.sangerbox.com). Genes that exhibited high expression
levels in the HFD group while demonstrating low expression in the ND group were
selected for further analysis. Those genes exhibiting
Following the identification of DEGs, enrichment analysis were conducted to gain
deeper insights into the functional implications of the data, including Gene
Ontology (GO) analysis across Biological Process (BP), Cellular Component (CC),
and Molecular Function (MF), utilizing the SangerBox website. Furthermore, the
Kyoto Encyclopedia of Genes and Genomes (KEGG) database was utilized to annotate
the primary signaling pathways associated with the differentially expressed genes
(DEGs). The results of the KEGG analysis were illustrated using a chordal graph
generated via the SangerBox website again. In the context of GO and KEGG
analyses, p
The ChEA3 website (https://maayanlab.cloud/chea3) was utilized to identify transcription factors (TFs) associated with differentially expressed genes (DEGs). A comprehensive list of TFs, detailing the interactions between TFs and DEGs in a node-to-node format, was retrieved from this platform. The Xiantao Academic website (https://www.xiantaozi.com/) was employed to generate a bar chart illustrating the TFs ranked by their count of associated DEGs exceeding 20. There were a total of 4296 candidate genes. Venn analysis was used to compare the 4296 Genes that exhibited high expression levels in the HFD group and 210 genes related to ECM which were selected from the genecards database, counting the overlapping and unique significant differential genes. These 51 overlapping genes were identified as candidate genes associated with ECM. Subsequently, Cytoscape software (version 3.10.2, Seattle, WA, USA) was used to construct a relevant network showcasing the top 10 TFs along with their corresponding DEGs [35].
After using TRIzol Reagent (15596018CN, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, we isolated total RNA from cultured cells or mouse GSM samples. The isolated genomic DNA was converted to cDNA using an all-trans Transgen reverse transcription kit (AH101-02, Zhongguancun Dongsheng International Science Park, Beijing, China). RT-PCR was conducted using the SYBR Green I (AQ131-01, TransGen Biotech, Beijing, China) method for mRNA expression analysis. Target gene expression was calculated with the 2-ΔΔCt method. The primer sequences used for amplification are shown in Table 1.
| Species | Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
| mouse | Collagen Type I Alpha 1 Chain (Col1a1) | CCCGAGGTATGCTTGATCTG | TCTTTGGGGGTTGGGACAGT |
| mouse | Collagen Type I Alpha 2 Chain (Col1a2) | TTGTGGATACGCGGACTCTG | CAGTGGGGCCCTTTCGTACT |
| guinea pig | Secreted Phosphoprotein 1 (SPP1) | GCTGCACGACAACAAACTCC | GACATGGCATTCTGTGGTGC |
| guinea pig | S100 Calcium Binding Protein A4 (S100A4) | TGCCTTGGATGTGATGGTGT | AAAGCAGCTTCATCCGTCCT |
| guinea pig | N-Myristoyltransferase 1 (NMT1) | AGATGATGGAAGGCAATGGGAA | CCACCTCGGATGTAGCTGTT |
| guinea pig | Vimentin (VIM) | TGAGATCGCCACCTACAGGA | GAGTGGGTGTCAACCAGAGG |
| guinea pig | Epiregulin (EREG) | CTGAGCGCTGGGGATAACTT | GGATGCAGGACGGAATCACA |
| guinea pig | Integrin Subunit Alpha 2 (ITGA2) | GCTGCTTTTAGCGCTCAGTC | CTGTTCACTTGAAGGACCGGA |
| guinea pig | Integrin Subunit Alpha 4 (ITGA4) | ACACACTGCGCTTCAGCAAT | GGGGAACATCCAACCCCAAA |
| guinea pig | Integrin Subunit Alpha 5 (ITGA5) | TGTTTGTATTCCCTGGGGGC | CGCTCCTCTGGGTTGAACAT |
| guinea pig | Integrin Subunit Alpha 6 (ITGA6) | GTTTGGAGCTCCGGGTACTT | ATCACATCAGGGGATGTGTCA |
| guinea pig | Matrix Metallopeptidase 2 (MMP2) | CACCTTAACACCCGGCTTCT | GGGAACTGTTGAAGGGGGAG |
| guinea pig | Matrix Metallopeptidase 3 (MMP3) also named as Stromelysin-1 (LOC100729101) | GAGTCTGAGACACCACCAGC | ATGCTTTTTGGGAAACCGGC |
| guinea pig | Matrix Metallopeptidase 9 (MMP9) | TCCAACTTCGACGCAGACAA | GTGATCTAAGCCCAGTGCGT |
| mouse | Actin Alpha 1 (Acta1) | CCAGAGCAAGCGAGGTATCC | GCCACACGCAGCTCATTGTA |
| guinea pig | Actin Beta (ACTB) | TGCTGCGTTACACCCTTTCT | ACAATCAAAGTCCTCGGCCA |
Mice gallbladder tissues and GSMCs were collected respectively and lysed by RIPA
lysis buffer. In a 4 °C centrifuge, 12,000 rpm for 10 min, after collect the
supernatant, that is the total protein solution. And then, the protein
concentration was established by a BCA kit. Protein samples were separated by
electrophoresis on a 10% SDS-PAGE gel and then transferred to PVDF membrane.
Blotted membranes were placed in a blocking solution of 5% non-fat milk for 30
min and then incubated overnight at 4 °C with anti-Collagen I, anti-Vimentin,
anti-Osteopontin, anti-Integrin-
Guinea pig gallbladder tissue was embedded in OCT compound, frozen, and cut into 20-µm-thick sections for AFM analysis and then fixed to an AFM sample holder. The morphology of the sample was measured using AFM (Bruker Dimension Icon, Billerica, MA, USA) in the ScanAsyst in air mode using a cantilever made from Ohm-cm Antimony(n) doped Si (RFESPA-75). The measurement mode was then changed to contact mode with the same cantilever, and the force curve was measured at five points in the observed morphology area. Spring constant of 2.8 N/m was obtained by the standard calibration method of Dimension Icon Scanning Probe Microscope (SPM). The deflection sensitivity of 128 nm/V was obtained by Thermal Tune method. The RFESPA-75 tip radius was 8.00 nm. The RFESPA-75 tip half angle was 18.0. The first 200 nm of the extension curves were fitted using NanoScope Analysis Software version 3.0 (Bruker, Billerica, MA, USA) assuming a Poisson ratio of 0.3 and using the Hertzian fit model [36].
The preparation of muscle strips was conducted as described previously [37]. In
brief, guinea pigs were killed by CO2 gas asphyxiation, and their
gallbladders were quickly removed. The fat and serous membrane layers were
removed. The gallbladder was placed in a petri dish containing an ice-cold
Krebs-Henseleit (Krebs) solution and then cut into 0.2
Analyses were performed using Prism 9.5.0 software (GraphPad Software, San
Diego, CA, USA) and SPSS version 27.0 software (SPSS, Chicago, IL, USA).
Continuous variables derived from RT-PCR and Western blot analyses are presented
as means
To explore the effects of a HFD on the ECM of the GSM, the gallbladders of mice in the HFD and ND groups were stained using standard histologic techniques (Fig. 1). The results of the H&E staining indicated that compared with that in the ND group, the muscle layer of the gallbladder in the HFD group was thickened and intertwined into a network of structural tissues. For further study, the gallbladder tissue was also examined after Masson and EVG staining. Compared with that in the ND group, the muscle layer of the HFD group observed under microscopy appeared to be reticulated with collagen filled in the middle of the muscle fibers. Compared with that in the ND group, the collagen in the HFD group was increased.
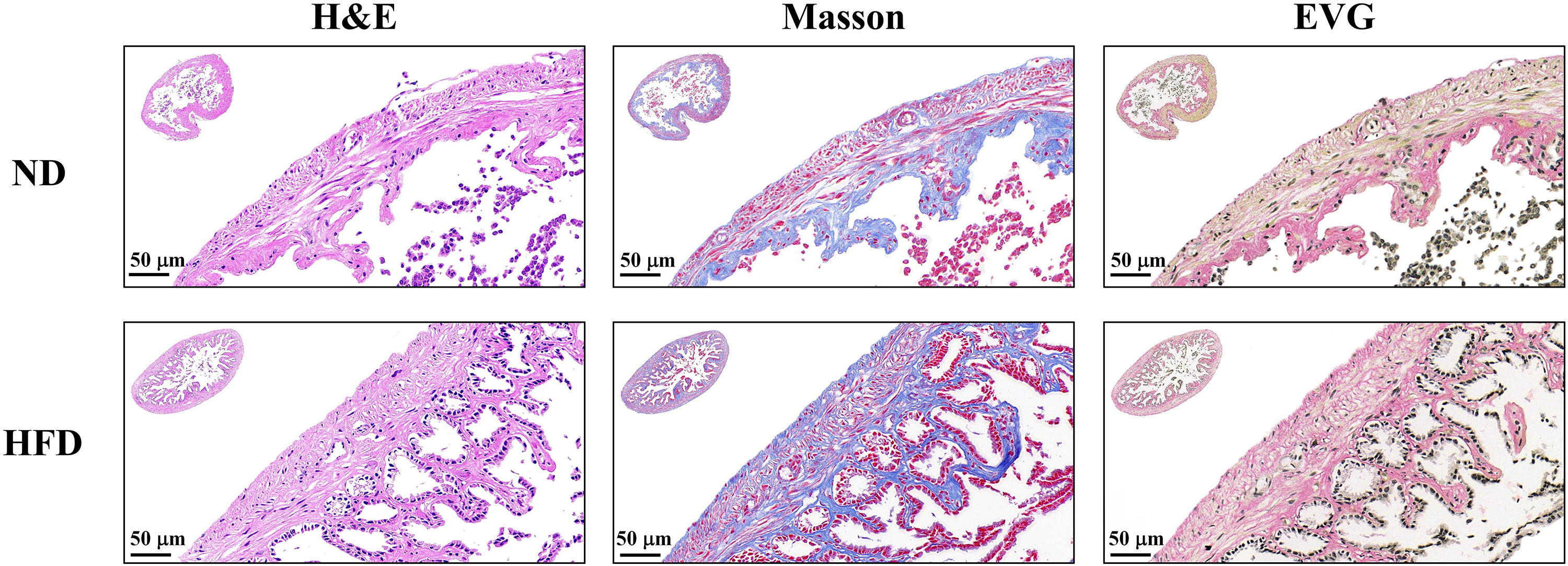 Fig. 1.
Fig. 1.
Histological staining of gallbladder tissue sections in mice fed a high-fat diet (HFD) or a normal diet (ND). Hematoxylin & eosin (H&E), Masson, and Elastica van Gieson (EVG) staining of gallbladder tissue sections. Collagen fibers appear blue, and muscle fibers appear red with Masson staining, whereas collagen fibers appear red and elastin appears black with EVG staining. Scale bar = 50 µm.
To investigate the changes in gene expression associated with ECM of gallbladder tissues induced by a HFD. Gallbladder tissues of mice from the HFD and ND groups were used for RNA Sequencing and bioinformatics analysis. The heat map representation of the analysis results reveals 51 DEGs that were significantly upregulated in the HFD group. Compared with those in the ND group, the expression levels of collagen, integrin family, and MMP genes were increased in the HFD group (Fig. 2A, Supplementary Table 2). The results indicated that 51 DEGs associated with ECM were successfully identified, predominantly localized to cell receptors associated with ECM.
 Fig. 2.
Fig. 2.
Expression and enrichment analysis of differentially expressed
genes in gallbladder tissue (C57BL/6 mice) between a high-fat diet (HFD) and a normal diet
(ND). (A) The heat map shows the most significant differences in extracellular
matrix (ECM) gene expression between the HFD and ND groups. Hfd_G1–3 indicates
tissue from three animals fed the HFD; Nd_G1–3, tissue from three animals fed
the ND. (B) Gene ontology (GO) analysis of upregulated genes in gallbladder tissue of
the HFD group compared with the ND group. (C) Kyoto Encyclopedia of Genes and
Genomes enrichment of DEGs. (D) Realtime quantitative polymerase chain reaction
analysis of the expression of Col1a1 and Col1a2 mRNA in
gallbladder tissues of the HFD and ND groups (n = 6). Data are expressed as the
mean
To further investigate the Gene Ontology and the signaling pathways associated with these DEGs, we performed an enrichment analysis based on them. The results of the GO enrichment analysis were particularly insightful, highlighting the most significantly enriched pathways including ECM organization (GO:0030198, BP), collagen-containing ECM (GO:0062023, CC), and ECM structural constituent (GO:0005201, MF) (Fig. 2B, Supplementary Table 3). To gain a more comprehensive understanding of the pathways enriched in these 51 highly expressed genes, we conducted KEGG enrichment analysis. The most significantly enriched pathways included ECM-receptor interaction (mmu04512), Focal adhesion (mmu04510), and the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway (mmu04151) (Fig. 2C, Supplementary Table 4). These pathways further underscore the critical role of the identified genes in ECM-related biological processes. The findings revealed that these 51 genes were significantly enriched in ECM receptor interactions and the PI3K-Akt signaling pathway. Results from GO and KEGG enrichment analyses suggest that Integrin family genes may serve as pivotal ECM receptors, facilitating the activation of the PI3K-Akt signaling pathway, which could influence phenotypic and functional alterations in GSMCs.
To validate the reliability of the aforementioned bioinformatics analysis results, we examined the expression of collagen genes, which are key components of ECM, using RT-PCR in gallbladder tissues from mice subjected to HFD and ND groups. Compared to the ND group, mRNA levels of Col1a2 gene were significantly elevated in the HFD group (Fig. 2D). Additionally, we employed Western blot to assess collagen deposition within tissues. The protein expression level of Collagen I was also markedly increased in the HFD group compared to that in the ND group (Fig. 2E). The findings from RT-PCR and Western blot analysis further substantiated that a HFD induces collagen deposition in gallbladder tissues.
To further substantiated the DEGs and enriched signaling pathways previously, which may implicate transcription factors (TF) and their potential mechanisms of action, we conducted an in-depth analysis of RNA sequencing data through various bioinformatics techniques, especially for TF and its connection with downstream genes. Total 57 TF genes, which the Count were greater than 20, were indicated to be involved in DEGs (Fig. 2F). Based on the number of DEGs associated with each TF, the top 10 TFs (RUNX2, PRRX1, TBX18, FOXS1, ERG, TCF21, FLI1, PRRX2, HEYL, and FOXC2) and their target DEGs were applied to create the regulatory network via Cytoscape software (Fig. 2G). The regulatory network consisted of 281 interactions between 10 TFs and DEGs associated with ECM (Supplementary Table 5). Based on the comprehensive analysis of KEGG enrichment analysis results, we found that the transcription factor RUNX2 may modulate by the PI3K/Akt signaling pathway and, either directly or indirectly via TIMP1, influence the expression of MMP2 proteins.
Overall, these findings indicated that the expression levels of collagen, integrin family members, and MMPs were all increased and that the PI3K-Akt signaling pathway was enriched in the HFD group. Moreover, ECM related transcription factors, such as RUNX2, may also play an important role in the dysfunction of GSM caused by HFD. A comprehensive understanding of the mechanisms involved, including cell receptors, signaling pathways, transcription factors, and downstream genes, may elucidate how HFD influence the structure and function of GSM via ECM, ultimately contributing to gallstone formation.
Due to the absence of prior reports regarding the phenotypic markers of GSMCs. In this study, we utilized synthetic phenotypic markers of vascular smooth muscle as a substitute for them. These synthetic phenotypic markers include Osteopontin (OPN), S100 Calcium Binding Protein A4 (S100A4), Vimentin, Epiregulin, and N-Myristoyltransferase 1 (NMT1) [39, 40, 41, 42]. The gene names they are dealing with are SPP1, S100A4, VIM, EREG, and NMT1. These markers have been employed for the comprehensive characterization of results obtained from RT-PCR and Western blot.
To investigate the effect of ECM components on GSMC phenotype transformation, guinea pig GSMCs (separated and identified as shown in Supplementary Fig. 1) were seeded in Matrigel containing 1 mg/mL or 2 mg/mL of collagen I, and the relative levels of their genes were analyzed by RT-PCR (Fig. 3A). The ECM of GSMCs seeded in 2 mg/mL collagen I showed significantly increased expression of genes related to the synthetic smooth muscle cell phenotype (Secreted Phosphoprotein 1 (SPP1), S100 Calcium Binding Protein A4 (S100A4), Vimentin (VIM), and Epiregulin (EREG)), integrin gene family members (Integrin Subunit Alpha 2 (ITGA2), Integrin Subunit Alpha 4 (ITGA4), and Integrin Subunit Alpha 6 ( ITGA6)), and MMPs (Matrix Metallopeptidase 2 (MMP2), Matrix Metallopeptidase 3 (MMP3), Matrix Metallopeptidase 9 (MMP9)) compared with GSMCs seeded in 1 mg/mL of collagen I (Fig. 3B–D). Next, we further validate the results of the three-dimensional cell culture using the Western blot. Compared with GSMCs seeded in 1 mg/mL of collagen I, the expression level of vimentin, ITGB1 and MMP2 in the GSMCs seeded in 2 mg/mL collagen I were significantly increased (Fig. 4A,B). Therefore, the results of RT-PCR and Western blot indicated that changes in the ECM may cause changes in the phenotype of GSMCs. There is currently a lack of research on the phenotypes of gallbladder smooth muscle.
 Fig. 3.
Fig. 3.
Extracellular matrix–induced phenotype transformation of
gallbladder smooth muscle cells (GSMCs) detected by realtime quantitative
Polymerase Chain Reaction (RT-PCR). (A) GSMCs were seeded in Matrigel containing
different concentrations of collagen I. Soft and hard Matrigels contained 1 and 2
mg/mL collagen I, respectively. After 48 h, the cells were harvested for RT-PCR
determination. (B–D) RT-PCR analysis of synthetic phenotype–related genes,
integrin family genes, and matrix metalloproteinase (MMP) genes expressed in
GSMCs cultured in Matrigel containing 1 or 2 mg/mL of collagen I (n = 6). Data
are expressed as the mean
 Fig. 4.
Fig. 4.
Extracellular matrix–induced phenotype transformation of
gallbladder smooth muscle cells (GSMCs) detected by Western blot. (A,B) Western
blot analysis of the expression of protein vimentin, OPN, ITGB1, MMP2, MMP9 in
GSMCs cultured in Matrigel containing 1 or 2 mg/mL of collagen I (n = 3). Data
are expressed as the mean
In addition, Compared with GSMCs seeded in 1 mg/mL of collagen I, the expression level of Akt in the GSMCs seeded in 2 mg/mL collagen I was also significantly increased (Fig. 4C,D). The result suggested that the PI3K-Akt signaling pathway may may serve as a crucial regulatory mechanism that mediates the effects of collagen deposition on GSMCs.
To investigate the effect of a HFD on the stiffness of the ECM and the
contractility of the GSM, we used the AFM nanoindentation method to investigate
the gallbladder muscle layer of guinea pigs in the HFD group compared with that
in the ND group (Fig. 5A,B). Compared with that in the ND group, the cantilever
deflection signal was significantly increased in the HFD group (1987
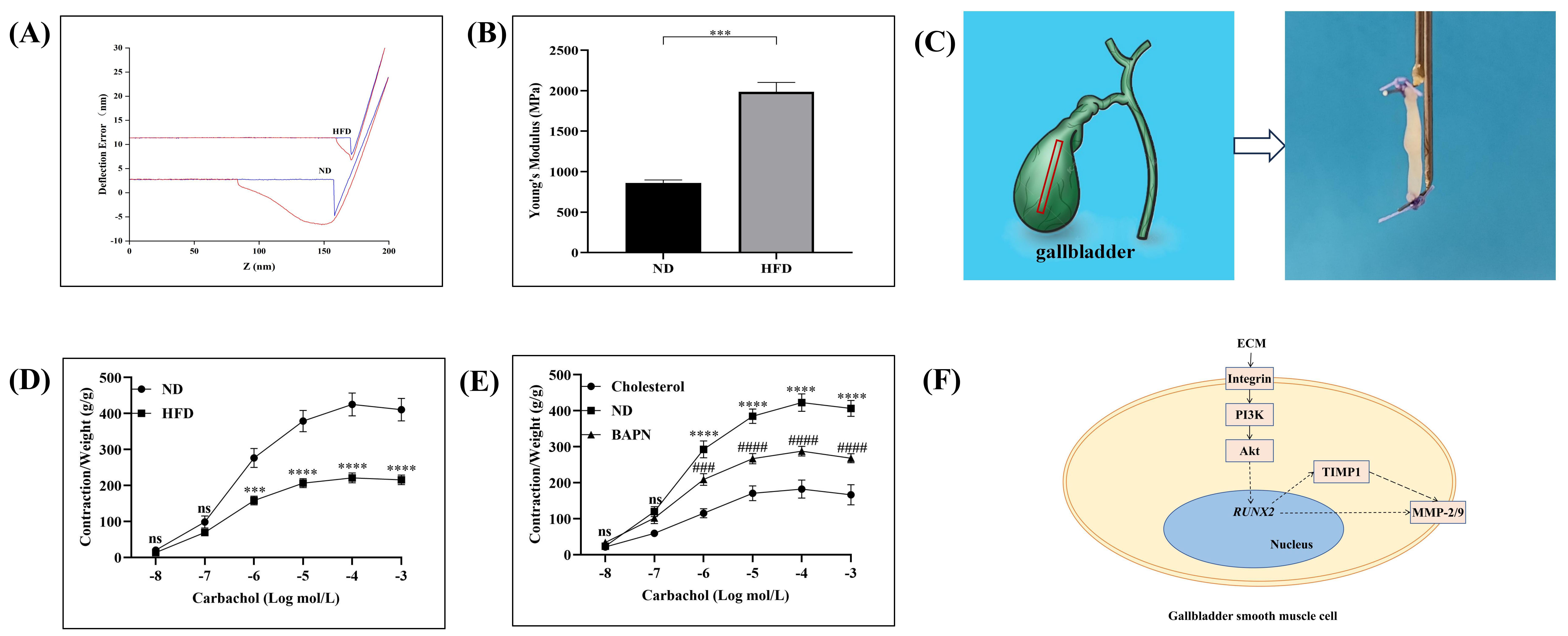 Fig. 5.
Fig. 5.
Effect of changes in the extracellular matrix on gallbladder
smooth muscle (guinea pig) tension. (A,B) The stiffness of the muscle layer of the
gallbladder was determined by using the atomic force microscopy (AFM)
nanoindentation method in the high-fat diet (HFD) and normal diet (ND) groups.
The deflection signal from the AFM cantilever was recorded and is shown by
representative approach (blue) and retraction (red) curves. ***p
We also prepared strips of GSM derived from the HFD and ND groups to measure changes in muscle contraction via a tension transducer in response to the application of agonists (Fig. 5C). Compared with that in the ND group, muscle contraction in response to stimulation with the agonist CCh was reduced in the HFD group (Fig. 5D). To investigate whether a HFD affected the function of the GSM through the ECM, a simple in vitro tissue model was used to simulate the possible changes in the gallbladder muscle layer after a HFD. The guinea pig gallbladder of the ND group was prepared as muscle strips, which were incubated with cholesterol [44]. We found that muscle tension was significantly reduced under cholesterol incubation compared to the control group (Fig. 5E). We then pretreated muscle strips derived from the gallbladder of guinea pigs in the ND group with BAPN and incubated the strips with cholesterol. Muscle contraction induced by the agonist CCh was partially restored after BAPN pretreatment compared with that for muscle strips incubated with cholesterol alone (Fig. 5E). Therefore, these results suggested that a HFD may induce GSM functional changes through alterations in the ECM. The suggested roles of the ECM in the phenotype transformation and contractility changes of the GSM and the signaling pathways potentially involved are illustrated in Fig. 5F.
The formation of gallstones is a complex process that is influenced by a number of factors, including genetic, cholesterol oversaturation in bile, gallbladder contractile dysfunction, rapid phase transition of cholesterol crystals, and intestinal aspects [45]. Owing to this complexity, the pathogenesis of gallstones remains unclear. In this study, we investigated the effects of an HFD on GSM phenotype and function as influenced by the ECM. Our main findings included the following: (1) a HFD caused disorders in the structure of the gallbladder’s muscle layer and ECM collagen deposition. (2) A HFD resulted in increased expression levels of collagen, integrin family, and MMP genes and enrichment of PI3K-Akt signaling pathway genes and related transcription factors such as RUNX2. (3) Alterations in the ECM induced the GSMCs to change from a contractile to a proliferative phenotype. (4) A HFD caused an increase in the stiffness of the ECM and a decrease in the contractility of the GSM.
When we histologically examined the gallbladder tissue of mice fed an HFD and those fed an ND, the results of H&E, Masson, and EVG staining showed that collagen was deposited in large quantities in the ECM of the gallbladder muscle layer in the HFD group. To our knowledge, this is the first time that it has been shown that an HFD can cause changes in the ECM composition of the gallbladder muscle layer and that a large amount of collagen is deposited. In comparing the findings presented in this study with previous studies on ECM changes in smooth muscle cells, several similarities were emerged. Related study has shown that the deposition of collagen in the ECM is closely related to fibrosis and degradation of tissue function [19]. In the context of muscle injury repair, a comparative morphological analysis of physiological changes in mice subjected to a HFD revealed significant tissue necrosis, immune cell infiltration, ECM remodeling, and fibrotic formation within the damaged muscle tissue [46]. The observed delay in muscle regeneration among HFD-fed mice can be attributed to impaired gene expression levels associated with ECM components, resulting in an increased incidence of fibrosis [47]. Furthermore, HFD exposure promotes ECM remodeling; notably, the upregulation of ECM constituents is closely linked to adipogenesis, indicating that fatty ECM deposition serves as a key indicator of obesity and metabolic dysregulation [48]. Prior research has established that alterations in ECM remodeling and stiffness are pivotal in the pathogenesis of various diseases such as fibrosis and smooth muscle dysfunction. This study further elucidates these concepts by highlighting the specific impacts of HFD on the ECM composition within GSMCs.
HFD is known to disrupt ECM homeostasis, leading to increased collagen deposition, altered gene expression, and signaling pathway activation. Our RNA-seq sequencing results showed that the expression levels of collagen, integrin family, and MMP genes were increased. Our PCR and Western blot analysis further confirmed that collagen I, one of the main components of the ECM, exhibited elevated expression levels in the gallbladder tissues of mice fed with a HFD. The DEGs were significantly enriched in multiple signaling pathways, including the PI3K-Akt signaling pathway. Meanwhile, the transcription factor most closely associated with these DEGs has been identified as RUNX2, which acts as a downstream target of Akt and functions either directly or indirectly as a transcriptional regulator for MMPs. The integrin/PI3K-Akt/MMP signaling pathway plays a pivotal role in mediating the interaction between smooth muscle cells and ECM. Integrins function as cell surface receptors that facilitate adhesion to ECM components, such as collagen and fibronectin [49]. Upon ligand binding, integrins initiate a cascade of intracellular signaling events by recruiting and activating PI3K, which catalyzes the conversion of PIP2 to PIP3 [50]. This second messenger, PIP3, subsequently recruits and activates Akt, thereby initiating downstream signal transduction processes [51]. RUNX2 serves as a crucial transcription factor involved in smooth muscle cell differentiation and ECM regulation. It can modulate the expression of genes associated with both ECM synthesis and degradation [52]. Following its activation, Akt enhances RUNX2’s transcriptional activity through phosphorylation of either RUNX2 itself or its upstream regulatory factors. By regulating TIMP1 and MMP2/MMP9 expression, either directly or indirectly, RUNX2 maintains a balance between ECM synthesis and degradation within smooth muscle cells [53]. Specifically, RUNX2 may inhibit excessive ECM degradation by promoting TIMP1 expression, thus preserving ECM stability and integrity [54]. Conversely, under certain conditions, RUNX2 can also stimulate MMP2/MMP9 expression to enhance smooth muscle cell migration and invasion capabilities [55]. The ECM is a dynamic structure in tissues and undergoes constant remodeling. Cells sense changes in their environment, which plays an important role in regulating their homeostasis, development, and pathology [56, 57]. To eliminate interference influence of non-GSMCs in gallbladder tissue, it is necessary to establish a model based on GSMCs obtained from primary culture to simulate the process of ECM changes caused by HFD and their impact on GSMCs. We have successfully established a in vitro model of three dimensional cell culture for RT-PCR and Western blot analysis to corroborate the findings from bioinformatics analysis. The results of RT-PCR and Western blot analyses demonstrated a significant upregulation of genes and proteins associated with integrin family members and MMPs in GSMCs cultured in a high collagen I environment. These findings are consistent with the results obtained from RNA sequencing and bioinformatics analyses. In addition, taking using the elevated expression of Akt protein in GSMCs cultured within a high-collagen environment as an example, the Western blot results corroborated the findings from bioinformatics analyses, not only regarding the expression differential genes but also concerning the enrichment signal pathways associated with DEGs. In summary, the integrin/PI3K-Akt/MMP signaling pathway embodies complex yet vital roles in regulating both smooth muscle cells’ functions as well as maintaining ECM homeostasis, thereby exerting profound effects on disease onset and progression.
Another important aspect highlighted in the study is that the
integrin/PI3K-Akt/MMP signaling pathway plays a critical role in the phenotypic
transformation of smooth muscle cells from a contractile to a synthetic
phenotype. Synthetic smooth muscle cells are characterized by increased
proliferation, migration, and ECM remodeling capabilities, which can further
exacerbate ECM stiffness and dysfunction [58]. The phenotype of smooth muscle
cells (SMCs) is dynamic rather than static. These cells can undergo phenotypic
transformation in response to various physiological and pathological stimuli. In
addition to changes in cell morphology, previous studies have shown that
biomarkers such as OPN and Vimentin were significantly upregulated in synthetic
SMCs [59, 60]. The expression of OPN was typically elevated in synthetic SMCs,
potentially serving as an adaptive mechanism following tissues injury [61].
Furthermore, the expression of Vimentin markedly increased during the transition
from contractile to synthetic SMCs, thereby enhancing cellular flexibility and
motility [62]. Notably, related research has indicated a close association
between the phenotypic transformation of SMCs and specific signaling pathways,
such as the integrin/PI3K-Akt signaling pathway which may be activated during the
transformation. Zhou et al. [63] demonstrated that overexpression of
TRIM65 can activate PI3K-Akt signaling, facilitating the transition of vascular
SMCs from a contractile to a synthetic phenotype. Integrins also play a pivotal
role in promoting both proliferation and migration of SMCs. For example,
activation of Focal Adhesion Kinase (FAK) by
Next, we investigated the effect of a HFD on the physicochemical properties of the ECM. Changes in physical and chemical properties may decrease smooth muscle contractility. The increase in the stiffness of the ECM we observed in the present study is consistent with an increase in collagen. As a scaffold protein in the ECM, its content and cross-linking intensity can affect the stiffness of the ECM [19]. Lysyl oxidase (LOX) is responsible for the cross-linking of collagen, and loss-of-function mutations in LOX interfere with the cross-linking of collagen, resulting in the softening of the ECM [65]. A simple in vitro experiment in the present study was conducted to simulate the gallbladder after an HFD. The reduced GSM tension measured in the presence of cholesterol was consistent with the observed reduction in muscle tension caused by an HFD. After treatment with BAPN, a specific inhibitor of LOX, the muscle tension reduction caused by cholesterol incubation was partially restored. Overall, these results indicated that changes in the ECM can affect the contractility of the GSM.
Mechanistic insights into the integrin/PI3K-Akt/MMP signaling pathway offer promising avenues for therapeutic interventions in various diseases. The integrin/PI3K-Akt/MMP signaling pathway represents a complex yet promising therapeutic target. By targeting specific components of this pathway, such as integrins, PI3Ks, Akt, and MMPs, therapeutic interventions can disrupt the signaling cascade that promotes disease progression [66]. Targeting integrins with antagonists such as peptides, antibodies, and small molecules disrupts their interactions, thereby inhibiting the migration and metastasis of cancer cells [67]. PI3K inhibitors impede phosphorylation processes that prevent Akt activation and subsequent downstream signaling pathways, demonstrating efficacy in clinical oncology trials [68]. Akt inhibitors can interfere with disease-promoting signaling either directly or through upstream modulators [69]. The modulation of MMPs, which are enzymes responsible for degrading the ECM, significantly influences cell migration and invasion [70]. However, although there are currently no clinical studies on the treatment of HFD induced gallstones via integrin/PI3K-Akt/MMP signaling pathways, ongoing research will help to develop more effective and targeted treatment strategies.
This study also has certain limitations. Firstly, only animal gallbladder samples were used in the experiment, and human gallbladder samples were lacking. We can obtain samples from the gallbladders of patients with gallstones for clinical purposes, but we cannot obtain normal gallbladder samples. Secondly, we did not knock down or knock out integrin and PI3K-Akt to establish a direct connection between the ECM and the phenotype and function of the GSM. We have only indirectly demonstrated the role of integrin/PI3K-Akt/MMP signaling pathway in the ECM affecting GSM phenotype and function. In addition, what we also need to note is that there are some limitations in these criteria, such as biological heterogeneity and technical noise, which may impact on the results of this study. Finally, this study only preliminarily explored the influence of HFD on the phenotype and function of GSM through the ECM. There is a lack of therapeutic methods to improve the ECM composition and restore the normal function of GSM. Further research into the integrin/PI3K-Akt/MMP signaling pathway may reveal key insights into gallstone formation and develop more effective treatments for gallbladder disease.
This study demonstrated a mechanism of action by which an HFD can alter the ECM composition of gallbladder muscle layer, activating the integrin/PI3K-Akt/MMP signaling pathway and affecting the phenotype and contractility of the GSM. This study informs understanding of the effects of an HFD on gallbladder dysfunction and the potential mechanism underlying these effects.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
ChaZ, XZ, ChoZ, BS: concept and design of this study. FW, JH, WG: conducted experiments and analyzed data. KZ, WG: animal model construction. FW, JP: interpreted data. XZ, FW, JH: drafted the manuscript. ChaZ, XZ, ChoZ, BS: reviewed data and proofread manuscripts. All authors participated in the revision of the manuscript and ultimately approved it. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All animal experiments were approved by the Biomedical Research Ethics Committee of Anhui University of Science and Technology (approval No. LZ2023-001), and all experiments were conducted in accordance with local and national guidelines and regulations.
The authors thank the Anhui Provincial Key Laboratory of Occupational Health, Anhui No. 2 Provincial People’s Hospital for support. We sincerely appreciate Thressa Smith, Ph.D. for revising the language of this manuscript.
This study was supported by the University Research Project of Anhui Province (2020ZR12925B003).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2912401.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
