- Academic Editors
†These authors contributed equally.
Background: Rapid progression and early metastasis remain the main cause of high mortality in epithelial ovarian cancer (EOC) patients. The objective of this study was to explore the mechanisms of EOC progression and detect the function of leucine-rich alpha-2-glycoprotein 1 (LRG1) in modulating the pathologic process. Methods: Ultracentrifugation was initially performed to extract exosomes from the urine samples of EOC patients and healthy female subjects. Mass spectrometry (MS) was employed to analyze differentially expressed proteins. Survival analysis was performed to examine the association between LRG1 levels and the prognosis of EOC patients. LRG1 silencing ovarian cancer cell lines were built and cell migration was further evaluated via wound healing and transwell assays. Immunoblot, immunofluorescence and immunohistochemistry analyses were performed. A subcutaneous tumor model was established to study the function of LRG1 in vivo. Results: Exosomal LRG1 was specifically expressed in urine samples of EOC patients and high LRG1 levels were significantly associated with poor prognosis. Function analyses showed that LRG1 was associated with ovarian cancer migration and progression. Mechanistically, LRG1 was significantly related to the focal adhesion kinase/protein kinase B (FAK/AKT) signaling pathway. Conclusions: LRG1 participated in progression and metastasis of ovarian cancer via activation of the FAK/AKT pathway probably.
Among malignant tumors of the reproductive tract, epithelial ovarian cancer (EOC) remains the leading cause of female mortality worldwide largely due to the rapid progression and peritoneal metastasis [1, 2]. No effective monitoring factors and treatment agents targeting progression and metastases in EOC have been achieved to date. Identification of critical molecular targets involved in progression and metastasis of ovarian cancer presents an urgent clinical challenge.
Exosomes are a subtype of extracellular vesicles (EVs) secreted by cells with a size range of ~40–160 nm (average ~100 nm) [3, 4]. Exosomes contain nucleic acids, proteins, lipids, and various other metabolites [5] and serve as crucial mediators of cellular communication. Exosomes participate in several biological processes [6], such as tumor generation, progression and chemoresistance [7, 8]. Accumulating evidence supports the utility of exosomes as promising clinical biomarkers for tumor diagnosis, disease monitoring and response to various therapeutic agents.
Leucine-rich alpha-2-glycoprotein 1 (LRG1), discovered in human serum in 1977, was initially characterized as a protein participating in inflammatory reactions [9, 10]. Studies have demonstrated LRG1 overexpression in a variety of human diseases [11], including some types of cancers such as gastric cancer [12], colorectal cancer (CRC) [9, 13] and breast cancer [14]. LRG1 is reported to play an important role in cancer growth and progression, with the potential as a chemotherapeutic target and a novel biomarker in some tumors. For example, LRG1 contributes to CRC cell proliferation and inhibits apoptosis through runt-related transcription factor 1 (RUNX1) activation, and may therefore serve as a promising predictive biomarker for monitoring disease recurrence [15]. Notably, LRG1 is highly expressed in ovarian cancer [16]. Smith et al. [17] found LRG1 peptides mainly existed in urine samples of ovarian cancer patients compared to the healthy controls. Wu J et al. [18] demonstrated LRG1 could be considered as a biomarker alone or in combination with CA125 for the diagnosis of ovarian cancer, although the underlying mechanisms were not established. More recent evidence suggests that serum-derived exosomes of LRG1 induce metastasis and serve as an effective biomarker for diagnosis and prognosis in colon cancer [19].
In this study, we used urine samples to extract exosomes for their easy and non-invasive method of collection. LRG1 was identified as a specific protein of urine-derived exosomes from ovarian cancer patients via mass spectrometer (MS). A subsequent study showed that LRG1 participated in the process of ovarian cancer cell migration in vitro and ovarian cancer progression in vivo. Mechanistic analyses revealed that LRG1 activated focal adhesion kinase/protein kinase B (FAK/AKT) pathway by elevating the phosphorylation level of FAK and AKT. The data from this current study advanced our understanding of the mechanisms associated with progression and metastasis and supported the utility of LRG1 or exosomal LRG1 as a therapeutic tool and biomarker for ovarian cancer.
Informed consent was obtained for experimentation with human subjects. This study was approved by the Ethical Committee of Xinhua Hospital affiliated with the Shanghai Jiaotong University School of Medicine (No. XHEC-D-2022-201). Urine samples from 10 patients with pathologically confirmed EOC and 10 healthy female subjects was collected. Patients with other medical or surgical disorders were excluded from the study. A total of 114 EOC primary tumor samples and 67 metastatic tissues were used for immunohistochemistry and survival analysis. All the samples were collected from January 2012 to December 2021 in the Xinhua Hospital.
Using urine samples from ovarian cancer patients and healthy female subjects,
exosomes were obtained and purified via ultracentrifugation, which is considered
to be the gold standard for exosome isolation [20, 21]. The procedure was
performed as described below. First, urine samples were centrifuged at 300
After determination of protein concentrations with the BCA kit (P0012, Beyotime,
Shanghai, China), 30
Ovarian cancer cell lines SKOV3 and HEY were obtained from the American Type
Culture Collection (ATCC, USA). The ovarian cancer cells were authenticated by short tandem repeat (STR) DNA profiling analysis and screened routinely for mycoplasma contamination. Cells were cultured in Dulbecco’s Modified
Eagle’s Medium (DMEM) at 37 °C under 5% CO
Silencing of LRG1 (experimental group) was achieved using shRNA technology.
Lentivirus carrying LRG1 shRNA was obtained from Bio-Link biological technology
company (Shanghai, China). LRG1 shRNA with the following sequences was used:
5
For immunoblot analysis, ovarian cancer SKOV3 and HEY cells were lysed on ice in
radioimmunoprecipitation assay (RIPA) lysis buffer (P0013B, Beyotime, Shanghai, China). The
acquired proteins were quantified with the BCA protein assay kit (P0010,
Beyotime, Shanghai, China), followed by denaturation, sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE), transmembrane and blocking. Membranes were incubated
overnight with primary antibodies (1:1000 dilution) at 4 °C and
washed with 1
In order to detect the function of LRG1 in regulating ovarian cancer cell
migration, transwell assays were performed. Ovarian cancer cells (5
The wound healing assay was conducted as follows: ovarian cancer cells were
seeded into 6-well plates (1
After fixation with 4% paraformaldehyde (E672002; Sangon Biotech, Shanghai, China), permeabilization with 0.3% Triton X-100 (A600198, Sangon Biotech) and blocking with 1% bovine serum albumin (BSA, A2153; Sigma, Saint Louis, MO, USA), cells or tissues were incubated with LRG1 (13224-1-AP, Proteintech, Chicago, IL, USA), p-FAK (Tyr397, ab81298, Abcam), p-AKT1 (Ser473, 9018, Cell Signaling Technology) antibodies at 4 °C overnight with 1:1000 dilution with PBS. Following three washes with PBS (SH30256-01, Hyclone, Logan, UT, USA), samples were treated with the corresponding secondary antibodies. Finally, images were obtained with the aid of confocal microscopy (Leica, TCS SPS, Wetzlar, Hesssian, Germany).
Following conventional dewaxing, endogenous peroxidase elimination, microwave repair and BSA blocking, human ovarian cancer tissues were incubated in LRG1 antibody (1:100 diluted with PBS, ab178698, Abcam) at 4 °C overnight. Next, sections were treated with secondary antibody, followed by immunohistochemical staining and alcohol gradient dehydration. Stained tissue slides were finally visualized by diaminobenzidine (DAB, G1212, Servicebio, Wuhan, China). The expression level of LRG1 was assessed by histochemistry score (H-score), which was determined as multiplicity of staining intensity and percentage of positively stained cells. The quartile of the multiplicity score was set as the boundary between low and high expression. The staining intensity was classified as negative (0), weak (light yellow, 1), moderate (brownish, 2), and strong (brown, 3); the proportion of positive cells was scored as 0 to 5% (0), 5% to 25% (1), 26% to 50% (2), 51% to 75% (3), and 76% to 100% (4). LRG1, p-FAK (Tyr397) and p-AKT1 (Ser473) were analyzed by the same method in murine tumor tissues.
Animal experiments were approved by the Ethics Committee of Xinhua hospital (No.
XHEC-F-2022-020). Ten four-week-old female BALB/c nude mice were obtained from
the Animal Experiment Center of Xinhua hospital. The mice were housed in cages at
25 °C and 50% humidity with a 12 h light/dark cycle, and provided with
sufficient food and water in the Animal Experiment Center of Xinhua hospital. The
water, food, cages, and other items in contact with the mice were sterile and
treated by aseptic technique within a certified biosafety cabinet. The 10 mice
were divided randomly into two groups (two cages), and every cage contained 5
mice. Ovarian cancer transplanted tumor models were generated by subcutaneous
injection of ovarian cancer cells into the back of nude mice. Five mice living in
the same cage were injected with 5
Statistical analysis was conducted using the Prism8 program (GraphPad software,
San Diego, CA, USA). The two sets of data were compared using Student’s
t-test, with p values
Given the critical role of exosomes as sources of tumor biomarkers, urine samples were obtained from 10 EOC patients and 10 healthy females. Exosomes were extracted via conventional ultracentrifugation. Exosomes were verified using three classic methods of identification, specifically, transmission electron microscopy, nanoparticle tracking analysis and immunoblot assay (Fig. 1A–C). It indicated that exosomes were mainly distributed in the range of 50–200 nm (Fig. 1A). To find key molecular markers of ovarian cancer, exosomal proteins were extracted and subjected to MS, which led to the identification of 442 differentially expressed proteins (Fig. 1D). Interestingly, there were 56 proteins detected only in tumor samples such as LRG1, SERPINA3, C4BPA, and so on (Supplementary Table 1). Based on GO analysis of the MS results, we verified that exosome proteins were significantly enriched (Fig. 1E). Moreover, LRG1 was specifically expressed in exosomes from the ovarian cancer group with the greatest intensity. In addition, we found that increased expression of LRG1 was associated with poorer prognosis in terms of both OS and PFS (Fig. 1F,G), suggesting that LRG1 might be an oncogene in ovarian cancer. Subsequent EOC tissue microarrays showed that the metastatic lesions contained higher levels of LGR1 than the primary ovarian cancer tissue (Fig. 1H,I). Moreover, we found that LRG1 was higher expressed in the metastatic lesions compared with the corresponding primary tumors (Fig. 1J). These findings support the utility of LRG1 or exosomal LRG1 as a biomarker for ovarian cancer diagnosis and prognosis and its potential role as a mediator in the process of cell metastasis.
 Fig. 1.
Fig. 1.Exosomal LRG1 is specifically expressed in urine samples of
ovarian cancer patients and LRG1 was associated with the poor prognosis. (A)
Urine-derived exosome concentrations and size distributions determined via
nanoparticle tracking analysis. (B) Representative transmission electron
microscopy micrograph of exosomes derived from urine samples. (C) Immunoblot
analysis of CD63, HSC70 and Alix in exosomes from urine samples. (D) Venn diagram
of mass spectrometry (MS) analysis of exosomal proteins derived from urine of
ovarian cancer patients and healthy subjects. (E) Results of gene ontology (GO)
analysis after MS assay. (F,G) Survival analysis of overall survival (OS) and
progression-free survival (PFS) in 62 LRG1 high-expressing and 52 LRG1
low-expressing samples. (H) LRG1 expression was examined by immunochemistry assay
in ovarian cancer tissue microarrays (n = 114). (I) LRG1 expression was examined
by immunochemistry assay in the metastatic lesions (n = 67). (J) Paired analysis
of the 67 metastatic lesions and the corresponding primary tumors. **p
To further verify the role of LRG1 in ovarian cancer metastasis, we performed in vitro experiments. First, the expression patterns of LRG1 in different ovarian cancer cell types were examined via immunoblotting. Cell lines displaying higher LRG1 expression were selected for subsequent LRG1 silencing study (Fig. 2A). Next, LRG1 shRNA were introduced into ovarian cancer SKOV3 and HEY cells to establish LRG1-silenced cell lines (Fig. 2B). Transwell and wound healing assays were performed to identify migration changes caused by LRG1 silencing. The migration cell count was significantly inhibited by 47.83% in SKOV3/LRG1-shRNA cells and by 48.10% in HEY/LRG1-shRNA cells (Fig. 2C,D). The migration range of SKOV3 cells was reduced by 64.07% in the LRG1-shRNA group and by 37.48% in HEY cells compared with the control (Fig. 2E,F). Our results demonstrated that LRG1 modulated the process of migration in ovarian cancer cells.
 Fig. 2.
Fig. 2.LRG1 participates in migration of ovarian cancer cells. (A)
Immunoblotting analysis of LRG1 in various ovarian cancer cell types. (B) The
effect of LRG1 silencing was tested by immunoblotting analysis. (C) Transwell
migration assay was performed to assess the migration ability induced by LRG1
silencing. (D) Statistical results of the transwell migration assay. (E) Wound
healing assay was utilized to measure the migration ability of ovarian cancer
cells. (F) Statistical results of the wound healing assay. The experiments were
technically repeated three times. Values were expressed as mean
While the above results indicated that LRG1 modulated migration of ovarian cancer cells, the underlying mechanisms remained to be established. Immunoblotting and immunofluorescence assays were performed, and FAK and AKT1 were found to be expressed at low phosphorylated levels in LRG1 silencing ovarian cancer cells (Fig. 3A–C), though their total levels were seldom changed. The activated phosphorylation of FAK (Try397) and AKT (Ser473) played important roles in tumor metastasis [23, 24]. In view of these findings, we concluded that LRG1 may be involved in metastasis formation through activation of the FAK/AKT1 pathway.
 Fig. 3.
Fig. 3.LRG1 regulates FAK/AKT axis. (A) Immunoblotting assay of
phosphorylation of FAK (Tyr397) and AKT1 (Ser473). The experiments were
technically repeated three times and the quantification of the protein bands was
performed using image J software. (B) Immunofluorescence assay of LRG1 and p-FAK
(Tyr397). (C) Immunofluorescence assay of LRG1 and p-AKT1 (Ser473).
4′,6-diamidino-2-phenylindole (DAPI) was used to stain cell nuclei. **p
To further validate the function of LRG1 in ovarian cancer, in vivo experiments were performed on 4-week-old female BALB/c nude mice. In a subcutaneous tumor model, LRG1 knockdown markedly inhibited tumor growth velocity (Fig. 4A,B) along with tumor weight (Fig. 4C). After tumors were dissected, the tissues were subjected to immunohistochemistry and immunofluorescence. Both of these two methods demonstrated that LRG1 silencing inhibited the expression of p-FAK (Try397) and p-AKT1 (Ser473) (Fig. 4D–F). The data obtained from mice tumors suggested that LRG1 participates in ovarian cancer progression by regulating the FAK/AKT axis in vivo.
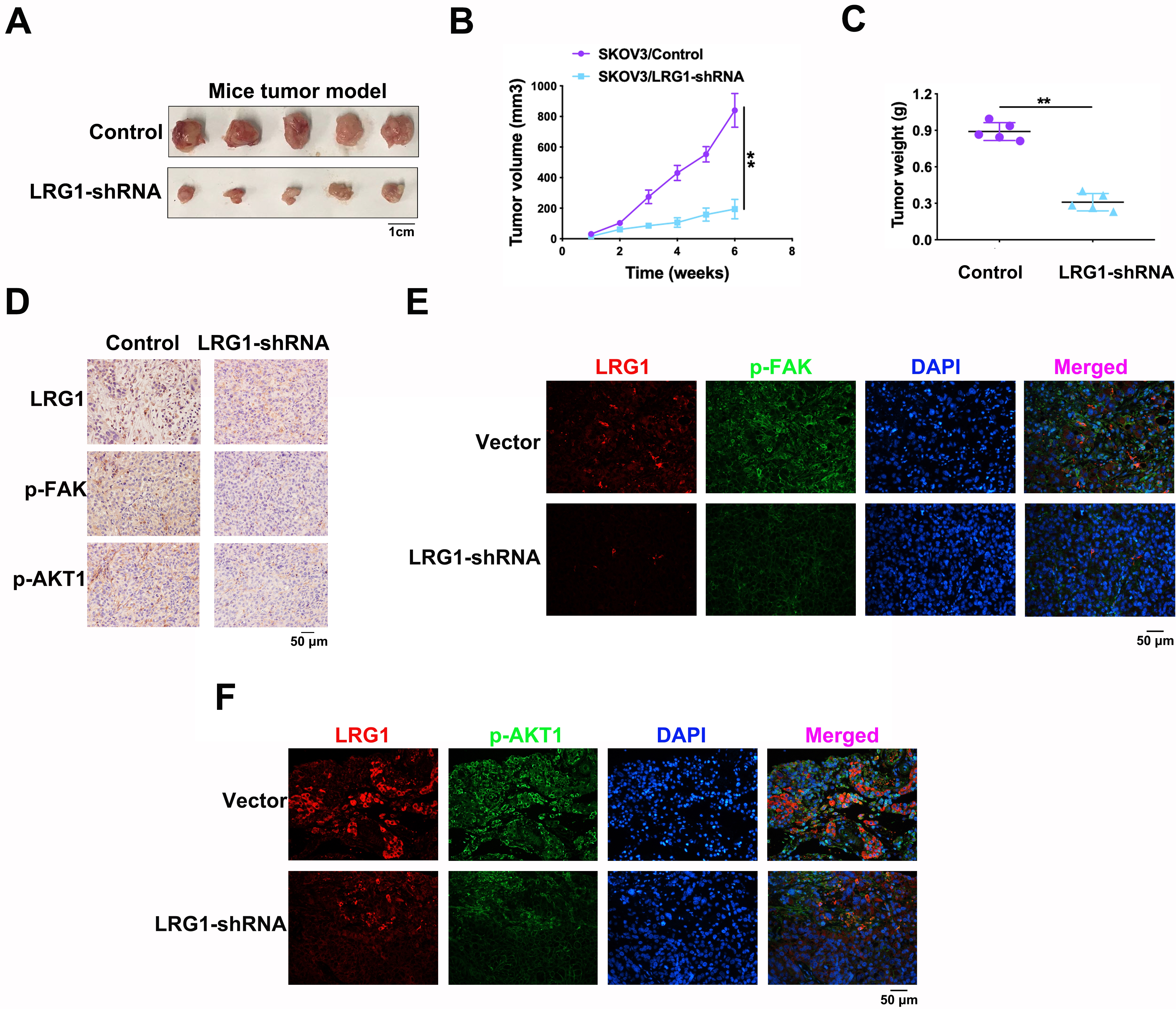 Fig. 4.
Fig. 4.LRG1 participated in ovarian cancer progression in
vivo. (A) Subcutaneous tumor model of ovarian cancer. (B) Tumor growth curve
indicated the changes of tumor volumes after SKOV3 cells were implanted
subcutaneously. (C) Tumors were weighed after they were dissected from the mice
bodies. (D) Immunohistochemistry analysis of LRG1, p-FAK (Tyr397) and
p-AKT1(Ser473) in nude mice subcutaneous tumor model. (E) Immunofluorescence
assay of LRG1 and p-FAK (Tyr397). (F) Immunofluorescence assay of LRG1 and p-AKT1
(Ser473). DAPI was used to stain cell nuclei. **p
In this study, we demonstrated that exosomal LRG1 was upregulated in the urine samples of patients with EOC and that LRG1 was associated with ovarian cancer progression and metastasis probably via activation of the FAK/AKT signaling pathway. To our knowledge, the potential involvement of the LRG1 in the pathogenesis of ovarian cancer has not been documented to date. Therefore, our experiments might provide novel insights into the regulatory mechanisms critical in the progression and metastasis of ovarian cancer.
Exosomes, a subset of EVs secreted by the majority of cells, have significant utility in cancer diagnosis and therapy [25, 26, 27]. For example, glypican-1 (GPC1)-positive exosomes and regulatory miRNAs (miR-96-5p and miR-149) have been identified as effective biomarkers for detection and therapeutic targets of CRC [28]. Exosomes serve as intercellular messengers via the circulatory system of all body fluids, including blood, urine, saliva and tears [29, 30, 31]. Urine samples present a good option for medical studies owing to their non-invasive and easy method of collection. Urinary exosomes have been recently shown to serve as biomarkers for prediction, surveillance and therapy of tumors involving the urinary system, such as prostate cancer [32, 33], renal cancer [34] and bladder cancer [35]. Accumulating evidence supports the utility of urinary exosomes as a biomarker in a number of tumor types other than tumors of the uropoietic system, such as breast cancer [36] and lung cancer [37]. Proteome analysis of urine exosomes from 10 ovarian cancer patients and 10 healthy individuals revealed specific expression of LRG1 only in urinary exosomes of ovarian cancer patients. Previous studies indicated that LRG1 participated in tumor vascularization and was considered as a therapeutic target for metastasis [38, 39, 40]. Our functional experiments revealed a role of LRG1 in the activation of ovarian cancer cell migration in vitro. Furthermore, in vivo experiments showed LRG1 participated in the process of tumor progression. Data from these collective studies may demonstrate a role for LRG1 in the induction of progression and metastasis of ovarian cancer.
The FAK/AKT signaling axis is a very important pathway in cancer progression [41, 42, 43]. Some studies demonstrated that activation of FAK/AKT pathway promoted the process of epithelial to mesenchymal transition to induce tumor progression and metastasis [44, 45]. Our results demonstrate that LRG1 silencing decreases p-FAK (Tyr397) expression and its downstream p-AKT1 (Ser473) to inhibit the tumor promotion. Kwan YP et al. [46] discovered that LRG1 promoted metastatic dissemination of melanoma through regulating EGFR/STAT3 signaling pathway. Ban Z et al. [47] indicated that LRG1 enhanced migration of thyroid carcinoma cells by activating MAPK/p38 signaling. Thus, we inferred LRG1 might induce ovarian cancer progression by other FAK/AKT-independent pathways, which need further study.
In summary, LRG1 sensitizes the FAK/AKT pathway to participate in ovarian cancer cell progression. As far as we know, this has not been previously reported. These results provide valuable insights into the regulatory mechanisms of ovarian cancer progression and metastasis and support the utility of LRG1 or exosomal LRG1 as a novel biomarker that may effectively aid in the diagnosis, therapy and prognosis analysis of ovarian cancer.
All data generated or analyzed during this study are included in this published article.
HS and XC conceived the study. DW and WX performed the experiments. DW analyzed the data. DW and HS wrote and revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethical approval for the study was obtained from Ethics Committee of Xinhua hospital. Approval Number of the use of human tissue samples was XHEC-D-2022-201. Approval Number of animal experiments was XHEC-F-2022-020.
Not applicable.
This work was supported by grants from Project of the Shanghai Municipal Health Commission (No. 20194Y0039 to Huizhen Sun) and the Natural Science Foundation of China (No. 82003053 to Xin Chen).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
