Veeva RIM
Regulatory Transformation
with Unified RIM
End-to-end regulatory information management on a single platform.
Learn about IDMP and best practices from BMS and GSK
Veeva RIM
Veeva RIM streamlines global regulatory processes on a single, cloud-based platform to improve visibility, data quality, and agility.
-
Speed to Market
Respond faster to changing regulations and increase process efficiency from submission planning to publishing.
-
Stronger Compliance
Ensure teams are developing reliable regulatory content with high data integrity.
-
Global Alignment
Coordinate regulatory efforts across HQ, affiliates, and partners within a single RIM system.
-
Unified and Connected
Tackle cross-functional business processes as part of the Veeva Development Cloud.
Veeva Registrations
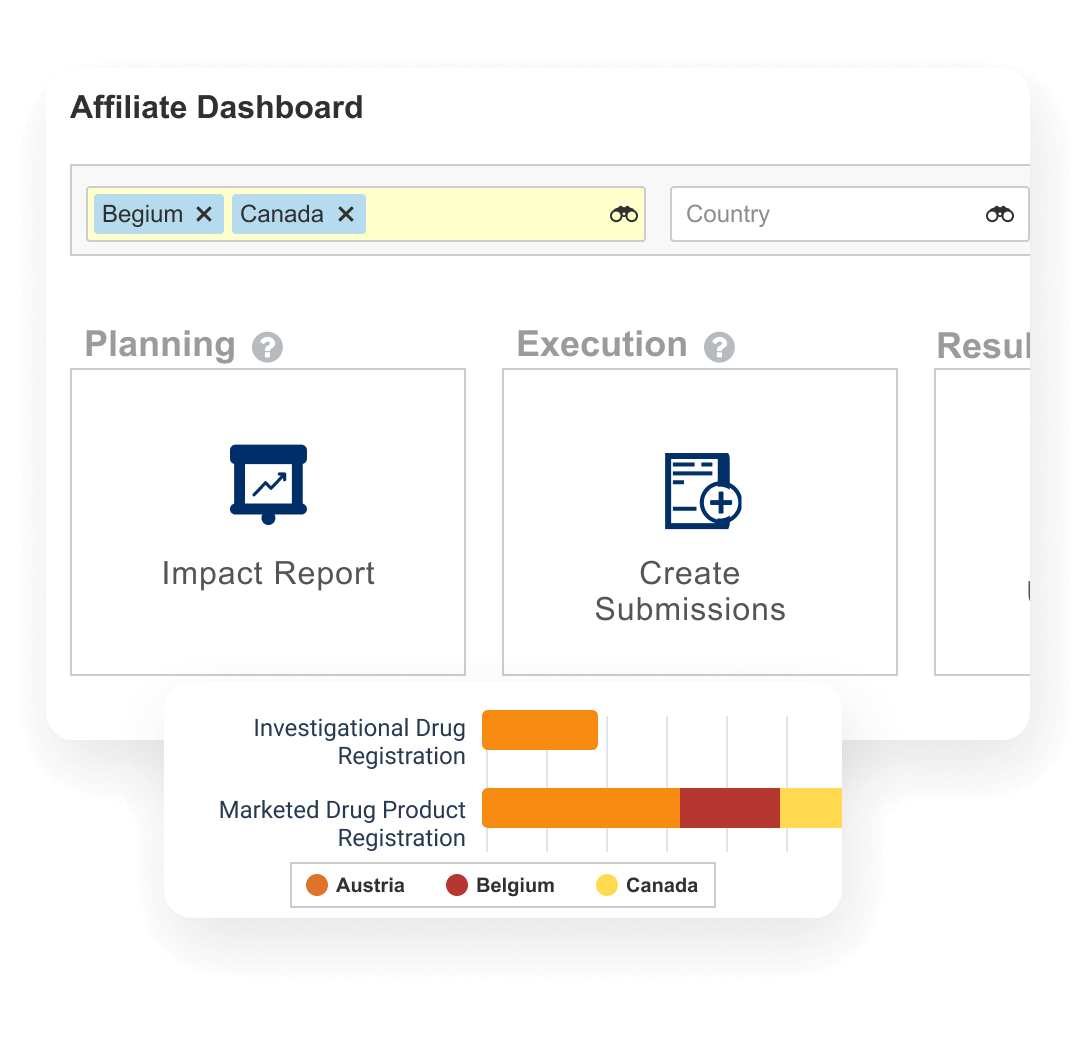
Veeva Registrations manages product registration data worldwide, including registration status, variations, and health authority interactions. Veeva’s flexible data model accommodates IDMP data points and will continue to support evolving regulatory data standards. Watch this short video on Veeva's approach to IDMP.
Veeva Submissions
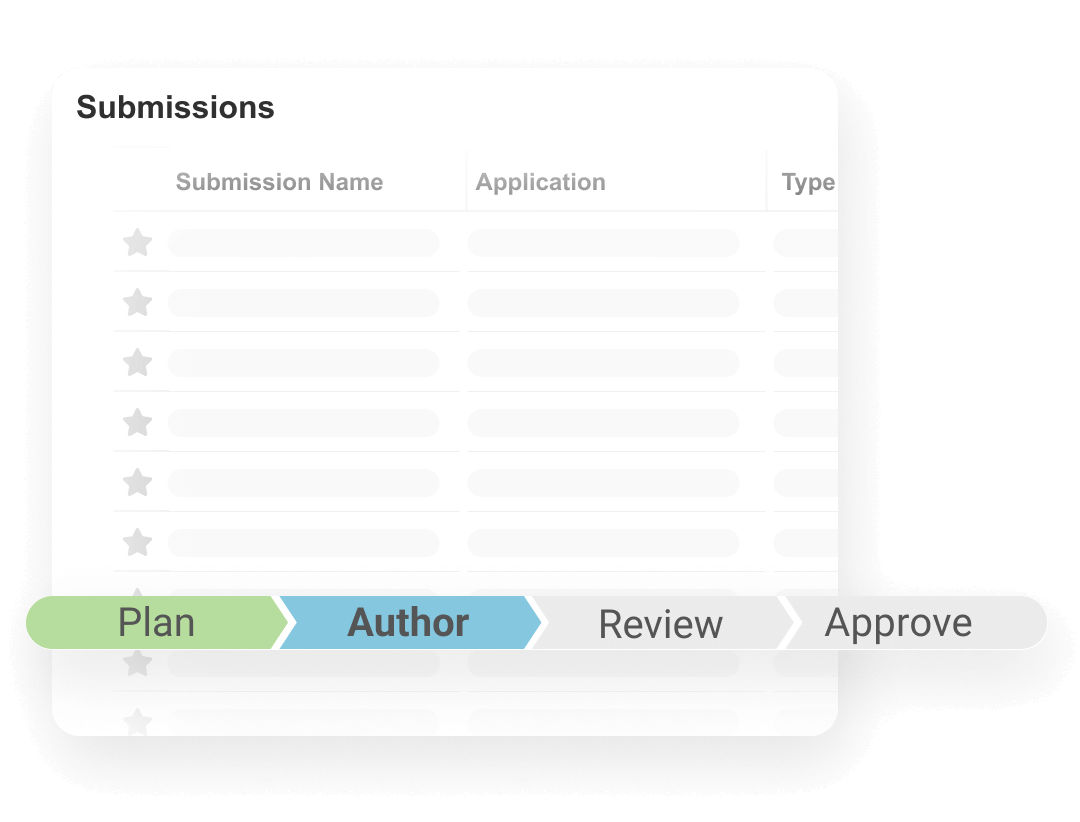
Veeva Submissions streamlines submission planning through approval by automating regulatory process steps. Users can create submission content plans, render submission-ready documents, and track submission status with complete traceability.
Veeva Submissions Publishing
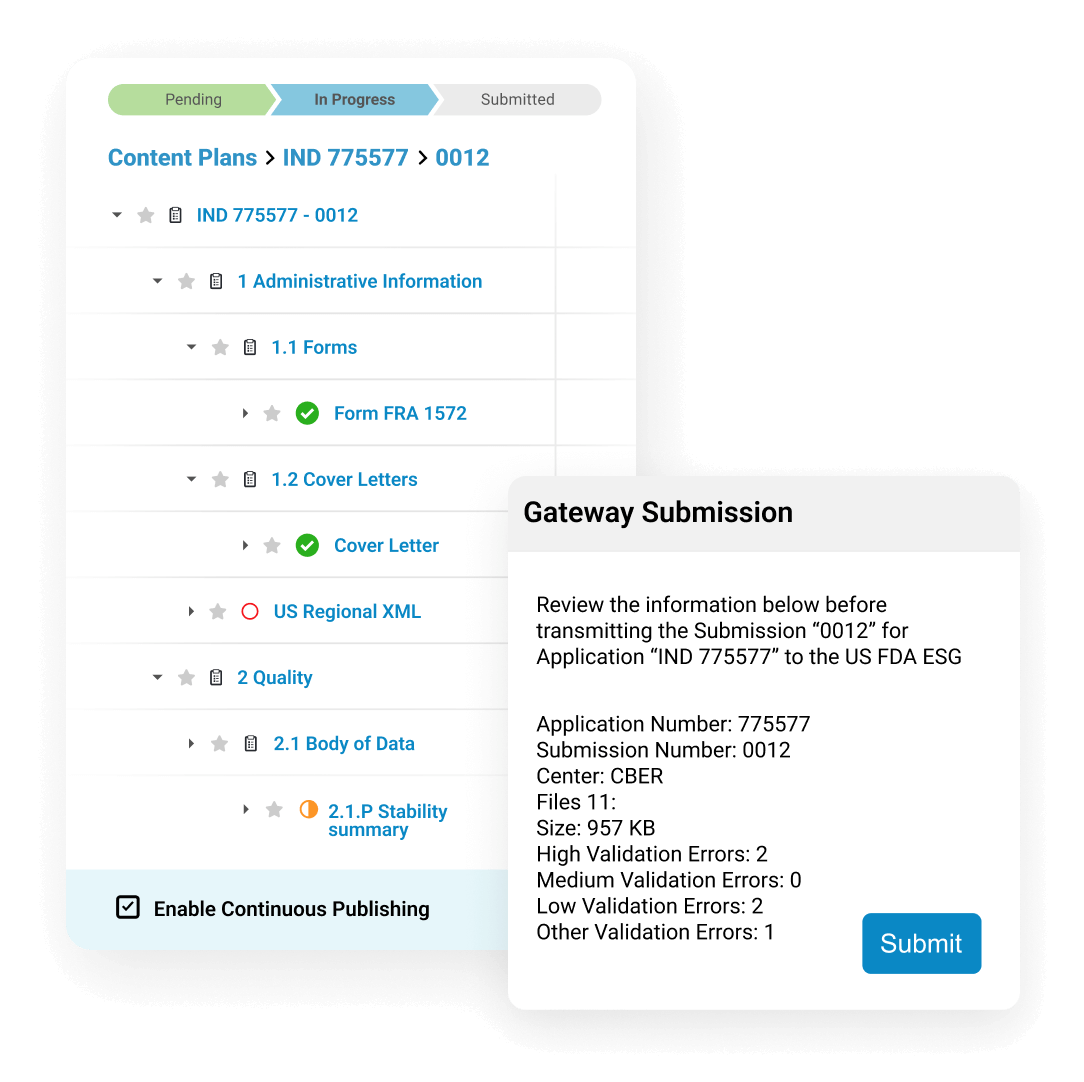
Veeva Submissions Publishing incorporates publishing functionality within Veeva RIM for end-to-end submission development. By employing a continuous publishing process to create, validate, and submit dossiers, users can dramatically speed submission delivery.
Veeva Submissions Archive
Veeva Submissions Archive stores a complete history of regulatory submissions securely in the cloud. Affiliates can download submissions or submission components for reuse in local markets and upload their submissions to local health authorities.

Veeva Regulatory Basics
For emerging biotechs, Veeva Regulatory Basics delivers Veeva's industry-leading regulatory applications, ready-to-use out-of-the-box with no implementation or maintenance costs.

Veeva Connections
Veeva Connections are Veeva-delivered integrations that seamlessly transfer data and documents. For regulatory teams, the Veeva RIM to Clinical Operations Connection enables users to automatically share product, study, and site information, the Veeva Quality and Veeva RIM Connection shortens the overall timeline from change control event creation to implementation, and the Veeva RIM to PromoMats Connection integrates compliance package generation for direct publishing to health authorities.
To see a full list of available Veeva Connections, visit the Veeva Development Cloud page.




