Veeva LIMS
Transform Quality Control
with a Unified Solution
Optimize QC labs to reduce complexity and increase right first time.
Announced 2021 Status Early Customers1-10

See how Veeva LIMS can modernize your QC operations
Overview
Optimize GMP manufacturing
quality control with Veeva LIMS
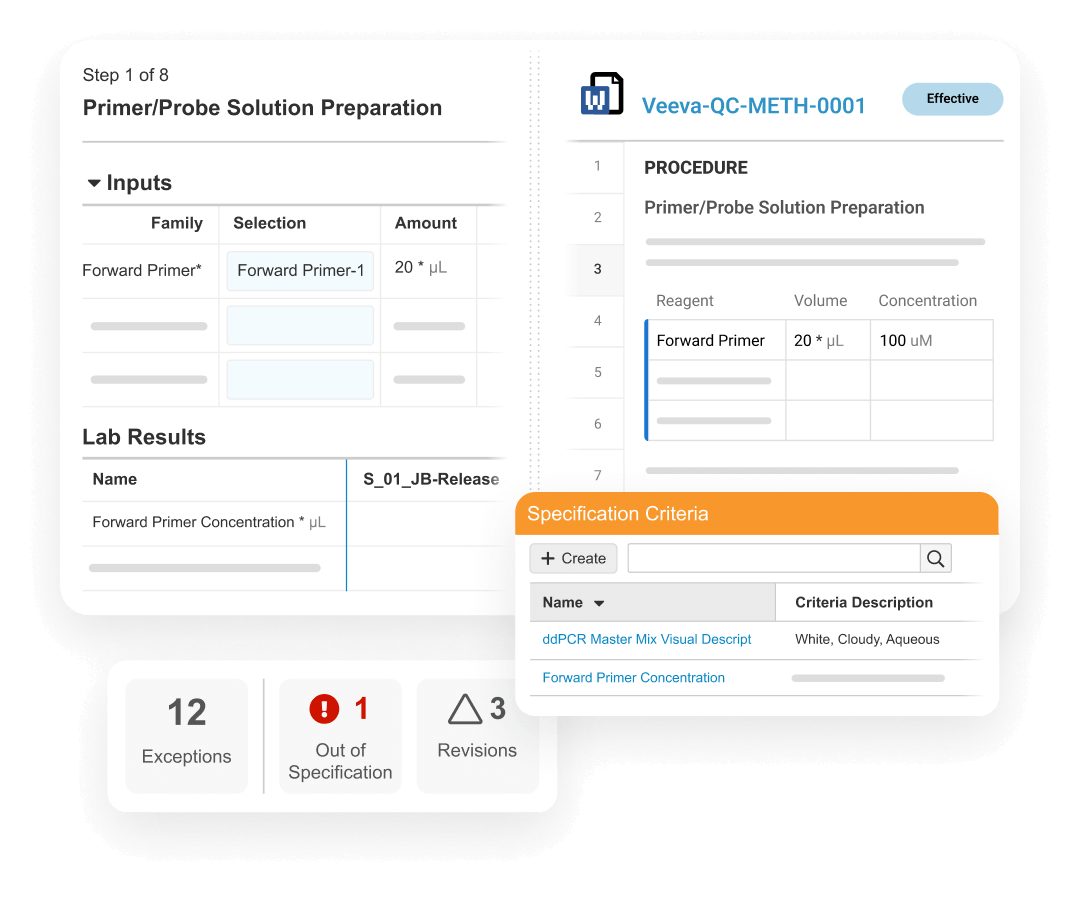
Veeva LIMS enables Quality Control to optimize batch release testing, stability study management, and environmental monitoring. It drives digital method execution, specification adherence, and review by exception to accelerate the release of product.
LIMS promotes compliance by verifying user qualifications from Veeva Training, displaying effective test method procedures from QualityDocs, and initiating lab investigations directly in QMS from out-of-specification (OOS) results.
Why Veeva LIMS
Faster, more agile quality control
Resources
Explore and Learn
Watch video
Why Veeva LIMS

Read case study
How Veeva LIMS is helping Forge Biologics scale QC for rapid growth

Read whitepaper
5 Misconceptions Keeping you from Modernizing QC

Read blog
How cloud and data are transforming QC

Read article
Three steps to unify a disconnected Biopharma Lab

Read blog
Modernizing QC strategy to achieve right-first-time manufacturing

Read blog
Breaking the status quo and embracing the redefinition of LIMS

Read blog
4 strategies for modernizing the manufacturing quality control lab

Read article
Getting lab data closer to the decision point keeps product release on track
