This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Single-cell RNA analysis reveals key immune mechanisms in lungs of pigs infected with reproductive and respiratory virus
Porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs is economically devastating for the global swine industry. The viral infection leads to reproductive disorder in sows and respiratory problems in infected newborn and growing pigs.
Unfortunately, high genetic variability of the virus and differing disease-causing strength or virulence hinders vaccine development and complicates disease management. Not much is known about the factors contributing to viral disease severity or the anti-viral immune responses.
Dr. Jun-Mo Kim, Associate Professor at the Department of Animal Science and Technology, Chung-Ang University, Korea, has focused his research efforts on filling this gap in understanding.
"Using a PRRSV infection model, our goal is to advance the comprehensive understanding of the infection and response mechanism in order to minimize industrial damage," states Dr. Kim.
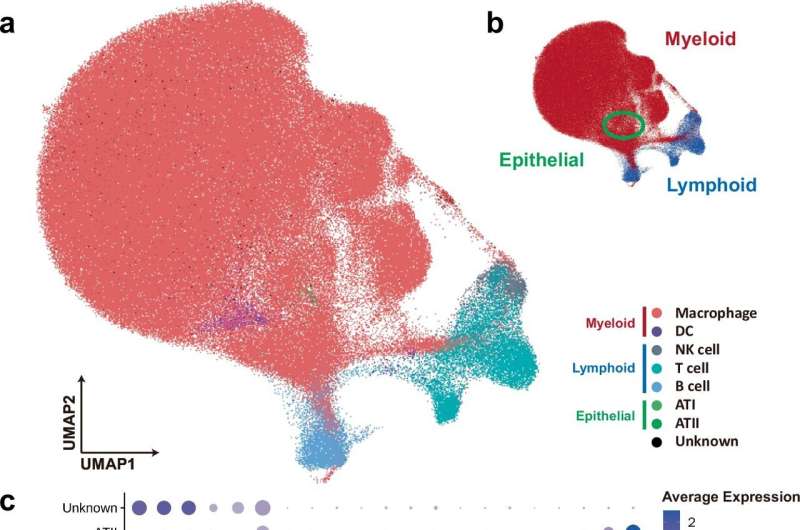
Recently, his group analyzed protein-coding RNA transcripts isolated from single cells and fluid collected from the lungs of PRRSV-infected pigs. They monitored immune cell alterations triggered by PRRSV strains of varying virulence. The paper is published in Nature Communications.
The study shows that high virulence PRRSV strain triggered early, severe lung damage and overall immune imbalance marked by significant reduction in macrophages. In contrast, the PRRSV strain of intermediate strength led to delayed lung damage with fewer immune alterations. Importantly, higher numbers of protective anti-inflammatory M2-like macrophages (SPP1-CXCL14high) were observed in less virulent infections, suggesting a potential role in promoting lung healing.
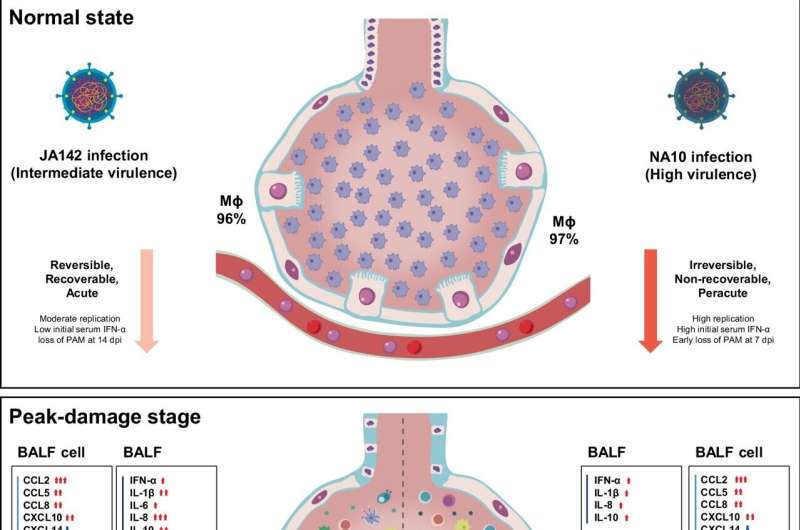
While fewer than 5% of macrophages were directly infected by virus, cell death and immune responses were widespread, indicating that extracellular vesicles or exosomes containing microRNAs released from the PRRSV-infected cells likely contributed to pathogenesis and cell death in non-infected bystander cells.
"Our study findings will aid the development of innovative therapeutic strategies with the potential to mitigate severe lung damage and promote efficient recovery in PRRSV-infected animals, paving the way for effective viral disease management," explains Dr. Kim.
In addition to the obvious impact on livestock health, global food security, and on securing the economy of the swine industry, the findings from this study may find a parallel in other human respiratory viruses and therapeutic strategies are likely to be applicable and effective broadly.
With these insights, researchers are one step closer to turning scientific discovery into real-world solutions that safeguard both animal and human health.
More information: Byeonghwi Lim et al, Single-cell transcriptomics of bronchoalveolar lavage during PRRSV infection with different virulence, Nature Communications (2025). DOI: 10.1038/s41467-024-54676-2
Journal information: Nature Communications
Provided by Chung Ang University