February 24, 2025 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Another novel virus found in bats with the potential to infect humans: HKU5-CoV-2
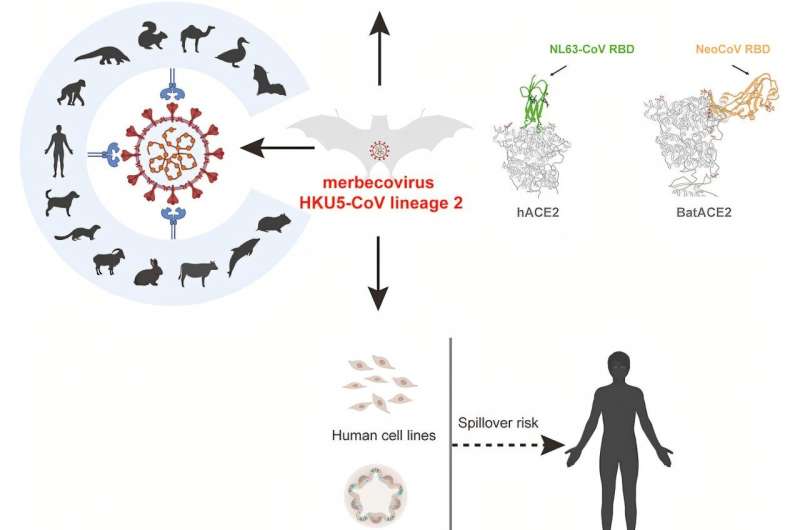
Research led by the Wuhan Institute of Virology has identified a distinct bat virus lineage capable of infecting human cells using the ACE2 receptor. The newly discovered merbecovirus, designated HKU5-CoV-2, was isolated from Pipistrellus bats collected in Guangdong Province, China.
In lab testing, the virus demonstrates broad host affinities across various mammalian species, though no evidence of human transmission has ever been reported.
Pipistrelle bats are found worldwide, from the American West to southern Europe and Asia, and are the most common bat throughout the UK. Small enough to fit inside a child's shoe, these small nocturnal mammals primarily feed on flying insects like mosquitoes and moths.
Merbecoviruses, a subgenus of betacoronaviruses, include MERS-CoV and several bat-derived coronaviruses. Historically, these viruses primarily use the DPP4 receptor for cell entry.
Analysis revealed HKU5-CoV-2's utilization of human ACE2, a receptor typically engaged by SARS-CoV, SARS-CoV-2, and HCoV-NL63. Previous research on HKU5-CoV lineage 1 showed limited ability to use human ACE2. HKU5-CoV-2's use of this receptor raises concerns over potential animal and human transmission.
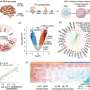
In the study, "Bat-infecting merbecovirus HKU5-CoV lineage 2 can use human ACE2 as a cell entry receptor," published in Cell, the team details the virus's identification, structural analysis, and infection mechanisms. Researchers screened Pipistrelle anal swab samples using pan-coronavirus PCR and sequenced six HKU5-CoV strains.
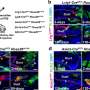
Phylogenetic analysis classified these viruses into a distinct lineage (lineage 2). Representative strain BtHKU5-CoV-2-441 shared 78.8%–78.9% genomic similarity with the HKU5 prototype strain. Structural differences in the receptor-binding domain (RBD) distinguished HKU5-CoV-2 from lineage 1 viruses.
Cryo-electron microscopy revealed that HKU5-CoV-2 binds human ACE2 through a unique interaction pattern. Mutagenesis analysis showed that single-point mutations in the RBD minimally affected binding, indicating that the virus's RBD can tolerate mutations without significantly affecting ACE2 binding. Functional assays confirmed HKU5-CoV-2's ability to infect human ACE2-expressing cells.
Authentic virus isolate BtHKU5-CoV-2-023 infected human respiratory and enteric organoids, with viral titers increasing by up to 2 log units. Infection was absent in ACE2 knockout cells and restored upon ACE2 reintroduction.
Protease dependency assays indicated that both TMPRSS2 and cathepsin L facilitate viral entry. The spike protein's S1/S2 and S2 cleavage sites were crucial for membrane fusion, though their cleavage efficiency was lower than that of MERS-CoV. Trypsin treatment enhanced viral entry and S protein cleavage.
Potential therapeutic interventions were evaluated. Monoclonal antibodies targeting the S2 subunit and the fusion inhibitor EK1C4 inhibited viral entry. Small molecules nirmatrelvir, remdesivir, and GC376 suppressed viral replication in cell culture. Unlike broad S2-targeting antibodies, SARS-CoV-2-targeting antibodies did not effectively neutralize HKU5-CoV-2.
Even with HKU5-CoV-2's ACE2 affinity and broad potential host range, its lower binding affinity and suboptimal S protein cleavage reduce the immediate risk of human spillover. While not an immediate threat, the virus's mutational potential for cross-species transmission and adaptation ability warrants continued surveillance.
No in vivo animal studies were conducted to test pathogenicity and transmissibility in a live host.
More information: Jing Chen et al, Bat-infecting merbecovirus HKU5-CoV lineage 2 can use human ACE2 as a cell entry receptor, Cell (2025). DOI: 10.1016/j.cell.2025.01.042
© 2025 Science X Network