This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Underlying mechanisms of age-related dysfunction in glands crucial to eye function identified

A team led by Mount Sinai researchers has identified stem cell populations and mechanisms underlying age-related degeneration in glands that are vital to eye function. The findings, published in Nature Communications on February 15, may lead to new therapeutic approaches for evaporative dry eye disease, a common condition in older people.
Meibomian glands, tiny oil glands along the edges of the eyelids, secrete lipid-rich meibum to prevent tear evaporation and protect the eye surface. Aging-related shrinkage of the meibomian glands may result, in part, from stem cell exhaustion and is associated with evaporative dry eye disease, a common condition that causes swollen eyelids, itchy eyes, or blurred vision. Symptoms may be lessened with warm compressions, artificial tears, and thermal pulsation, but these treatments are only partially effective.
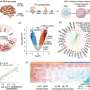
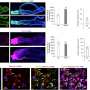
The researchers identified markers for stem cell populations that maintain distinct regions of the meibomian glands, and uncovered the hedgehog (Hh) cell-cell signaling pathway, which is broadly important in development and disease, as a key regulator of meibomian gland stem cell proliferation and tissue regeneration. They found that increased Hh signaling is a hallmark of human meibomian gland carcinoma, a rare and aggressive cancer of the eyelid.
Furthermore, the team discovered that aged glands show decreased Hh signaling and decreased epidermal growth factor receptor (EGFR) signaling, as well as impaired innervation and a loss of collagen in niche fibroblasts, suggesting that changes in both glandular epithelial cells and their surrounding microenvironment contribute to age-related degeneration. These discoveries suggest that targeting Hh and EGFR signaling to stimulate stem cell activity in the meibomian glands could be a potential therapeutic option to treat evaporative dry eye disease.
"Despite the prevalence of dry eye disease, the stem cells and molecular mechanisms that control homeostasis of the meibomian gland, and are impaired in aging, are poorly understood," said senior author Sarah E. Millar, Ph.D., Dean for Basic Science of the Icahn School of Medicine at Mount Sinai, the Lillian and Henry M. Stratton Professor of Gene and Cell Medicine, Director of the Institute for Regenerative Medicine, and Director of the Black Family Stem Cell Institute. "We hope that our work will eventually result in new, more effective therapies for this very common condition."
The researchers used a mouse model system for most of their analyses because the meibomian glands of mice have a similar structure to those in humans and, like the human glands, display decreased size and a reduced number of secretory cells in aging. The team carried out analyses including single nuclear RNA sequencing, in vivo lineage tracing, ex vivo live imaging, and genetic gain- and loss-of-function studies. Additionally, the researchers analyzed gene expression in normal human eyelid samples and in human meibomian gland carcinoma.
Dr. Millar said future work will include preclinical studies to determine whether small molecules that activate Hh and EGFR signaling can rescue age-related meibomian gland degeneration. Researchers from the Johns Hopkins University, University of Michigan, and the University of Pennsylvania contributed to this study.
More information: Xuming Zhu et al, Identification of Meibomian gland stem cell populations and mechanisms of aging, Nature Communications (2025). DOI: 10.1038/s41467-025-56907-6