This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Finally solved: How the body's endocannabinoids spread through the brain
Marijuana-like endocannabinoids produced by your own body—how do they travel through the brain? It turns out that these fatty messenger molecules are transported in fatty vesicles. This surprising discovery contrasts with how traditional messenger molecules move. Dopamine and serotonin, for instance, travel as free-floating molecules between nerve cells.
"This is possibly a new form of communication between nerve cells in the brain," says chemist Mario van der Stelt.
Van der Stelt suspects that other fatty messenger molecules might move through the brain in the same way. This insight could lead to new treatments.
"The body's own 'marijuana' plays a role in pain and other neurological conditions. Now that we know how it moves, we can look for ways to influence its function," he explains.
Why did it take so long for scientists to uncover how one type of endocannabinoid, 2-AG, is transported? The problem with 2-AG was that it could not be tracked directly.
"Because it is a fatty substance, you can't simply see it under a microscope," Van der Stelt says. Standard measurement methods were ineffective because they destroyed the cells, making it impossible to track the substance over time.
The research is published in the journal Proceedings of the National Academy of Sciences.
Glowing cells as the key
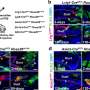
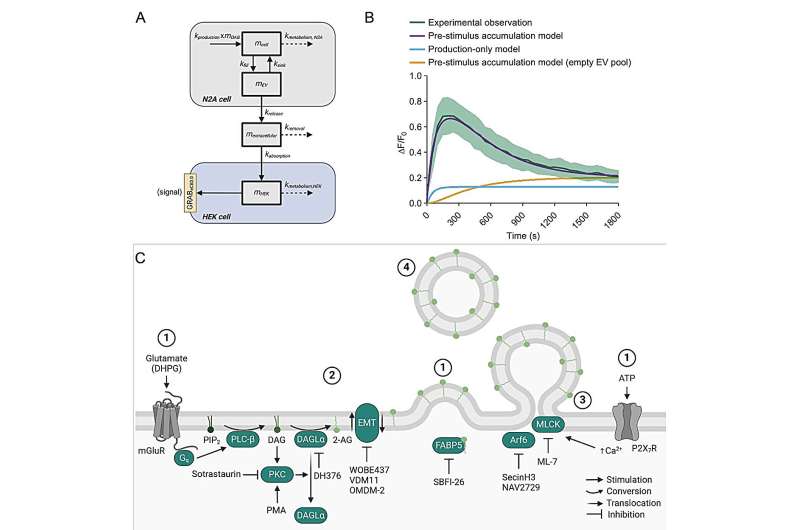
The breakthrough came when Chinese researchers developed a smart sensor. This sensor uses cells that light up when they detect 2-AG from a neighboring nerve cell. For the first time, this made it possible to observe 2-AG's movement in real time. This discovery laid the foundation for four years of research, culminating in the new publication, which marks the final piece of Verena Straub's Ph.D. research.
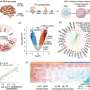
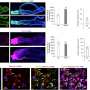
Thanks to this sensor, Straub discovered that 2-AG is transported in vesicles. She tested this by isolating and analyzing the vesicles. She found that when she blocked 2-AG production, vesicles still formed but no longer contained 2-AG. Conversely, when she prevented vesicle formation, the amount of 2-AG decreased. On average, each vesicle contained about 2,000 2-AG molecules.
To confirm the accuracy of their model, the researchers tested their findings in brain tissue in collaboration with a US-based group. They found indications that the same process occurs in intact brain tissue. Additionally, together with Coen van Hasselt's team, they developed a mathematical model that could only explain the observed signals if 2-AG was indeed transported via vesicles.
More information: Verena M. Straub et al, The endocannabinoid 2-arachidonoylglycerol is released and transported on demand via extracellular microvesicles, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2421717122. On bioRxiv: www.biorxiv.org/content/10.110 … 23.614520v1.full.pdf