Surgery-induced neuroinflammatory transcriptional programs in medial prefrontal cortex of mice during early phase of perioperative neurocognitive disorders
- Published
- Accepted
- Received
- Academic Editor
- Tiziano Balzano
- Subject Areas
- Genomics, Molecular Biology, Neuroscience, Cognitive Disorders, Surgery and Surgical Specialties
- Keywords
- Surgery, Cognitive dysfunction, RNA sequencing, Neuroinflammation, Medial prefrontal cortex
- Copyright
- © 2024 Tang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Surgery-induced neuroinflammatory transcriptional programs in medial prefrontal cortex of mice during early phase of perioperative neurocognitive disorders. PeerJ 12:e18664 https://doi.org/10.7717/peerj.18664
Abstract
Patients receiving anesthesia and surgery may experience cognitive dysfunction, memory deficits, and mental disturbances, which are referred to as perioperative neurocognitive disorders (PND). The function of the medial prefrontal cortex (mPFC) is disrupted during early phase of PND. To gain insight into the mechanisms of PND, we collected mouse mPFC tissues 6 h post-surgery and performed RNA sequencing analysis. In total, 178 differentially expressed genes (DEGs) were identified, including 105 upregulated and 73 downregulated genes. Bioinformatic analysis highlighted the significant enrichment of these DEGs in several immune-related biological processes and signaling pathways, suggesting that pronounced neuroinflammatory transcriptional programming in the mPFC was evoked during early phase of PND. Interleukin-6 level increased in both serum and mPFC, while the mRNA levels of Il-6, Tnf-α, and Il-1β remained unchanged. Taken together, our findings suggest that a distinct and acute neuroinflammatory response in the mPFC is evoked after peripheral surgery, which might play a key role in the development of PND.
Introduction
After anesthesia and surgery, some patients experience issues in concentrating, learning and memory impairments, and executive dysfunction, which can last for weeks or months (Moller et al., 1998; Evered et al., 2018). Perioperative neurocognitive disorders (PND) occur in 4.7–23.8% patients undergone surgery (Berian et al., 2018; Silva et al., 2021), leading to various adverse outcomes including prolonged hospitalization, increased morbidity and mortality, decreased quality of life, and an increased long-term risk of Alzheimer’s disease (Robinson et al., 2009). However, the underlying mechanisms of PND remain poorly understood.
Associative fear learning constitutes a defensive motivational system evolved to safeguard against environmental threats, involving stages such as acquisition/encoding, consolidation, retrieval, and extinction (Johansen et al., 2011). In PND models, mice exhibit a diminished response to associative threats following surgery, and fear conditioning is a widely used behavioral paradigm (Sun et al., 2023; Ma et al., 2024). Neuronal activity in the medial prefrontal cortex (mPFC) is essential for fear memory (Courtin et al., 2013; Agetsuma et al., 2023). Synaptic transmission is impaired in a subregion of the mPFC after surgery (Sun et al., 2023). Furthermore, the functional connectivity within the dorsal hippocampus-mPFC circuit is disrupted after surgery (Chen et al., 2022; Ma et al., 2024). The activities of mPFC-amygdala projections are dysregulated acutely after surgery (Sun et al., 2023). However, the molecular mechanisms underlying early degeneration of the mPFC remain unclear.
Considering the implications of mRNAs dysregulation in neurological disorders, investigating its role in PND pathogenesis is crucial. However, peripheral surgery-induced transcriptional responses in the mPFC during early phase remain unknown. In this study, we identified distinct immune response-related features in the mPFC of mice during early phase of PND by analyzing global transcriptomic changes and evaluating the levels of several proinflammatory cytokines.
Materials and methods
Animals and experimental design
A total of 61 male C57BL/6J mice (10–14 week-old; 25–30 g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and acclimated for 2 weeks. All mice were housed in ventilated cages (485 × 200 × 200 mm; 4–5 mice per cage) in standard conditions (12-h light/12-h dark cycle, lights on at 8 am; temperature, 22–24 °C; humidity, 55 ± 10%) with ad libitum access to water and food. Mice were tagged and randomly allocated to each group before any procedure. For open-field test, 10 mice were used in control and surgery groups; for fear conditioning test, 11 mice were used in control and surgery groups; for RNA sequencing (RNA-seq) and quantitative real-time polymerase chain reaction (qRT–PCR), three mice were used in control and surgery groups; for enzyme-linked immunosorbent assay (ELISA), 6–7 mice were used in control and surgery groups. Since whole blood and mPFC tissue collection were required for this study, mice were euthanized at the conclusion of the experimental period. Euthanasia was performed using 1% pentobarbital sodium (50 mg/kg, intraperitoneal injection). All animal experiments were approved by the Animal Care Committee of the First Affiliated Hospital at Zhejiang University School of Medicine (approval number: 2022-1435) and were conducted in compliance with the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery of tibial fracture
We used a clinically relevant mouse model of tibial fracture. The postoperative changes in these mice mimic certain cognitive impairments commonly observed in humans after routine orthopedic surgery, while exhibiting no apparent impact on sensory function and motor activities during behavioral tests (Xiong et al., 2018; Xiang et al., 2022). Mice underwent aseptic open tibial fracture surgery with intramedullary fixation under general anesthesia, as previously described (Xiang et al., 2022). Briefly, general anesthesia consisted of induction with 3.0% isoflurane followed by maintenance with 2.0% isoflurane in 30% FiO2. To expose the tibia, a median incision was made on the disinfected left paw. A 0.38-mm needle was inserted into the tibial medullary cavity, followed by osteotomy of the tibia at the junction of the middle and distal thirds. The fixation needle was left in place, trimmed flush with the tibial cortex, and the skin was sutured. The entire procedure from induction of anesthesia to the end of surgery was completed within 15 min. Mice were allowed to recover spontaneously from anesthesia. Throughout the surgery and recovery, body temperature was maintained at 37 °C using a heating pad. Analgesia (30 mg/kg tramadol) was subcutaneously administered after induction of anesthesia and prior to skin incision. Mice in the control group received identical anesthesia and analgesia but underwent no surgical procedures.
Open-field test
An open-field test was conducted to evaluate the spontaneous locomotor activity and anxiety levels in mice 24 h post-surgery. The apparatus consisted of a 50 cm × 50 cm open arena with 50 cm high walls and a floor divided into 25 equal squares. Briefly, mice were placed individually in the center of the apparatus and allowed to habituate and explore freely for 10 min. The total distance traveled and time spent in the center were automatically recorded using ANY-maze behavior tracking software (Stoelting Co., Wood Dale, IL, USA). The arena was thoroughly cleaned with 75% ethanol between trials.
Fear conditioning test
The contextual and cued fear conditioning test is a behavioral paradigm used to assess learning and memory. Freezing behavior, defined as complete immobility except for breathing, is a typical natural response to fearful situations. The extent of fear learning was recorded in a fear-conditioning chamber (Shanghai Xinruan Information Technology Co. Ltd., Shanghai, China). A decrease in freezing time indicated memory impairment.
Training
Mice were habituated to handling for 3 days before the behavioral tests. On the training day, mice were individually placed in the conditioning chamber and allowed to explore freely for 2 min. Subsequently, an auditory cue (80 dB, 400 Hz, conditional stimulus) was presented for 30 s and co-terminated with a 2-s foot shock (0.5 mA, unconditional stimulus). Three tone-foot shock parings were delivered at an inter-trial interval of 15 s. Mice were kept in the training chamber for another 2 min before being returned to their home cages.
Testing
Three days after the training session, mice were returned to the original conditioning chamber for 4 min without any tone or foot shock. Freezing behavior during this session was scored to assess contextual fear memory. After 6 h, mice were placed in another chamber with altered stripes and background odor, and were allowed to explore for 2 min. Mice were then exposed to an auditory stimulus (80 dB, 400 Hz) for 2 min, and the freezing duration was recorded to evaluate cued fear memory. The time spent freezing as a fraction of the total time spent in the chamber was recorded using a video-tracking system.
RNA-seq analysis
Total RNA was extracted from the mPFC of mice of the Control and Surgery groups (three mice in each group) using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and digested with RNase-Free DNase to remove residual DNAs. RNA quality was assayed on an Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and all samples sequenced exhibited an RIN > 8.0. Then, the poly (A) mRNA was enriched by oligo (dT)-attached magnetic beads and followed by fragmented into small pieces using fragmentation reagent. The RNA fragments were copied into first-strand cDNA using reverse transcriptase and random primers. Then second-strand cDNA was synthesized using the prepared second-strand synthesis reaction system. These cDNA fragments then had the addition of a single “A” base and subsequent ligation of the adapter. Library construction and sequencing were performed using a BGISEQ500 platform (BGI-Shenzhen, China). All raw data were deposited in the National Center for Biotechnology Information Sequence Read Archive database (accession ID: PRJNA1077729). The sequencing data were filtered using SOAPnuke (v1.5.2), and clean reads were mapped to the mouse reference genome (mm10) using HISAT2 (v2.0.4). Gene quantification was performed using RSEM (v1.3.1), and expression was quantified as fragments per kilobase of transcript per million mapped reads (FPKM). DESeq2 (v1.4.5) was used to identify differentially expressed genes (DEGs) between the groups. The adjusted p-value, termed Q value, was calculated by controlling false discovery rate. Fold changes (FC) of DEGs were determined based on the FPKM values. DEGs were identified using the criteria of |log2FC| ≥ 1 and Q value ≤ 0.05. To gain insight into phenotypic changes, Gene Ontology (GO, http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/) enrichment analyses of the annotated DEGs were performed. GO and KEGG terms were ranked by Q value, and a Q value ≤ 0.05 served as the cutoff criterion. The statistical power of this experimental design, calculated in RNASeqPower (An online implementation of RNASeqPower is available at https://rodrigo-arcoverde.shinyapps.io/rnaseq_power_calc/) is 0.83. There are six biological and technical replicates used to achieve the claimed statistical power.
qRT–PCR
RNA was reverse-transcribed into cDNA using the PrimeScriptTMRT Reagent Kit (Takara, Shiga, Japan), in accordance with the manufacturer’s instructions. qRT–PCR was performed using a QuantStudio5 thermocycler (Thermo Fisher Scientific, Waltham, MA, USA) and the SYBR Premix EX TaqTM II kit (Takara, Shiga, Japan) following the manufacturer’s instructions. Amplification was performed starting with an initial denaturation step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 31 s, in 10 μL reaction volume. All samples were amplified in triplicate, and the mean value was used for subsequent calculations. The fold changes in mRNA expression were calculated using the 2−ΔΔCt method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The sequences of primer pairs for the target genes are listed in Table S1. All PCR experiments and analyses adhered to the MIQE guidelines.
Measurement of cytokine levels
After mice were deeply anesthetized, whole blood was collected via left ventricular puncture. The blood samples were centrifuged at 3,000×g for 15 min at 4 °C, and serum was separated and immediately stored at –80 °C until analysis. Subsequently, mice were transcardially perfused with ice cold saline, and the mPFC tissues were dissected on ice under microscopic observation as previously described (Xu et al., 2016), immediately frozen in liquid nitrogen, and stored at –80 °C for further processing. Specifically, 1-mm-thick coronal brain sections were prepared by using a mouse brain mold. The mPFC tissue was then identified and collected with a biopsy punch based on the mouse brain atlas (Paxinos & Franklin, 2019), targeting the region approximately 2.3–1.3 mm anterior to the bregma, encompassing the cingulate, prelimbic and infralimbic subregions. The mPFC tissues were homogenized in NP-40 lysis buffer containing protease and phosphatase inhibitors (Beyotime Biotech, Shanghai, China), and were centrifuged at 12,000×g for 10 min at 4 °C. The supernatant was collected for analysis. Total protein content in the supernatant was quantified using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). A commercially available ELISA kit for detecting Interleukin (IL)-6 level in mice (R&D Systems, Minneapolis, MN, USA) was used according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (v7.0; Prism Software Inc., San Diego, CA, USA). Data are expressed as mean ± standard error of the mean (SEM). Comparisons between two groups were performed using an unpaired two-tailed student’s t-test, assuming equal variance. No data points were excluded from the statistical analysis. P < 0.05 was considered statistically significant.
Results
Cognitive decline after surgery
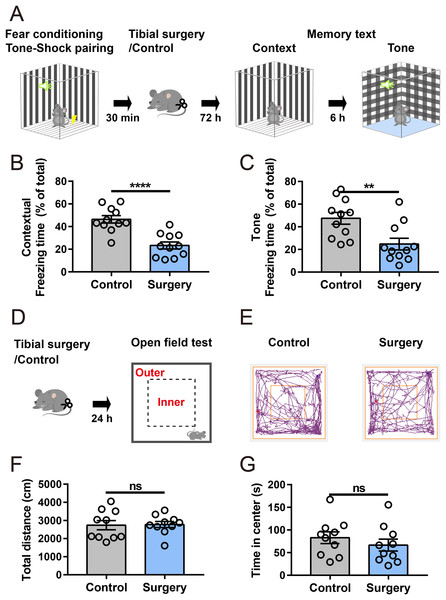
An aseptic model of tibial fracture surgery was established to investigate the underlying mechanisms of PND. Cognitive performance was assessed using a fear conditioning paradigm (Fig. 1A). Compared to that of the anesthesia-matched control group, the surgery group exhibited a significant decrease in freezing time in contextual and cued fear memory tests (Figs. 1B and 1C), suggesting that fear memory performance was impaired after surgery. In a separate cohort, in the open-field test (Figs. 1D and 1E), there was no significant difference in the total distance traveled between the control and surgery group (Fig. 1F), indicating spontaneous locomotor activity was not impaired after surgery. Additionally, time spent in the center zone of the open field was comparable between the two groups (Fig. 1G), suggesting no substantial difference in anxiety-like behaviors.
Figure 1: Tibial surgery leads to cognitive decline.
(A) Experiment paradigm, cartoon depicting the paradigm of contextual and cued fear conditioning behaviors. (B and C) Percent of time spent in freezing behavior (n = 11 mice/group). (D) Experimental paradigm, cartoon depicting the open field test. The open field is divided into 5 × 5 square bins, with 16 outer bins defined as the outer zone and the remaining area as the inner zone. (E) Representative tracings of the open field activity in control and surgery mice. (F) Total distance traveled in the open field and (G) Time spent in the center of the open field during test (n = 10 mice/group). Data are represented as mean ± SEM, ****P < 0.0001; **P < 0.01; ns, not significant.Transcriptome profile in the mPFC
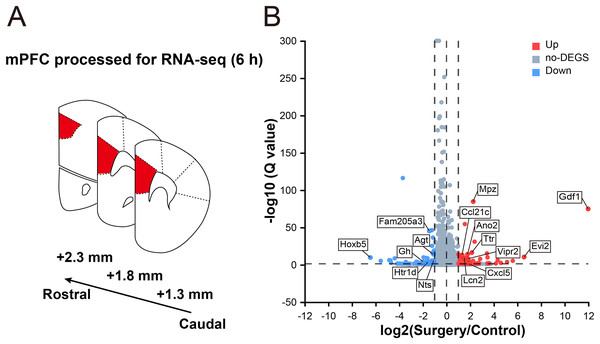
To reveal the surgery-induced changes in the mPFC at the transcriptional level, RNA-seq analysis and DEG screening were performed on six samples collected 6 h after surgery (three mice in each group, Fig. 2A). The sequencing generated, on an average, 66.90 million clean reads with a clean read ratio of 93.02%. From the mouse reference genome, 18,029 mRNAs were identified, corresponding to 202,923 genes.
Figure 2: Transcriptomic changes in the mPFC in mice at 6 h after surgery.
(A) Schematic coronal section of rat brain illustrating the mPFC according the rat brain atlas (approximately 2.3–1.3 mm anterior from the bregma). (B) Volcano plot illustrating DEGs between the surgery and control groups. Several main DEGs are annotated. Upregulated genes are labeled in red, downregulated in blue, and the rest in gray. |log2FC| ≥ 1, Q value ≤ 0.05 used as screening threshold, n = 3 mice/group.In total, 178 genes were differentially expressed between control and surgery mice (Table S2), and 105 were upregulated (log2FC ≥ 1, Q value ≤ 0.05), while 73 were downregulated (log2FC ≤ –1, Q value ≤ 0.05) (Fig. 2B). These findings indicated that significant reprogramming of gene expression in the mPFC was acutely induced after surgery.
GO enrichment analysis
To gain insight into the functions of these DEGs, GO analysis was performed, and the DEGs were categorized into three distinct ontologies such as biological process (BP), cellular component (CC), and molecular function (MF).
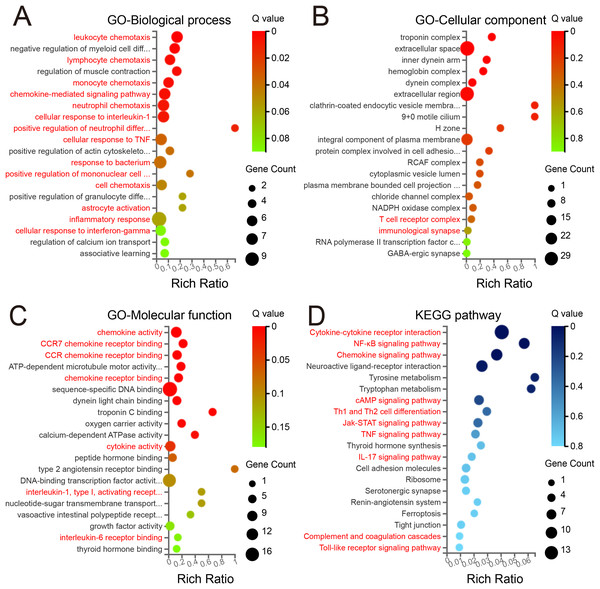
In the BP category (Fig. 3A), among the 20 top enriched terms, the majority were related with immune processes, including leukocyte chemotaxis, lymphocyte chemotaxis, monocyte chemotaxis, chemokine-mediated signaling pathway, neutrophil chemotaxis, cellular response to IL-1, positive regulation of neutrophil differentiation, cellular response to tumor necrosis factor (TNF), response to bacterium, positive regulation of mononuclear cell migration, astrocyte activation, inflammatory response and cellular response to interferon-gamma (IFN-γ), which suggest a significant involvement of immune activity. In the CC category (Fig. 3B), the enriched terms included NADPH oxidase complex, protein complex involved in cell adhesion, T cell receptor complex and immunological synapse. Regarding the MF class (Fig. 3C), several enriched GO terms pertained to inflammation-related binding processes, including chemokine activity, CCR7 chemokine receptor binding, CCR chemokine receptor binding, chemokine receptor binding, cytokine activity and IL-6 receptor binding. These findings suggest an active inflammatory response in the mPFC region.
Figure 3: GO and KEGG enrichment analyses of DEGs.
The 20 top enriched GO terms are shown in the biological process (A), cellular component (B), and molecular function (C). (D) The 20 top KEGG pathway enrichments with DEGs. Red text denotes terms related to immunological processes. The ordinate represents the Q value, while the abscissa shows the enrichment ratio. Dot size represents the count of genes, and color indicates the Q value. A Q value ≤ 0.05 was considered significantly enriched.KEGG pathway analysis
To further elucidate the biological functions of the identified DEGs, KEGG pathway enrichment analysis was performed. Surprisingly, the three top significantly enriched pathway terms were cytokine–cytokine receptor interaction, nuclear factor kappa beta signaling pathway, and chemokine signaling pathway (Fig. 3D). Moreover, several pathways associated with neurogenic diseases (neuroactive ligand–receptor interaction, cell adhesion molecules, and tight junction) were among the 20 top enriched terms. Of note, several inflammation-related pathways involved in signal transduction were also enriched, including cAMP, Jak-STAT, TNF and IL-17 signaling pathways, Th1 and Th2 cell differentiation, complement and coagulation cascades, and Toll-like receptor signaling pathway. Together with GO analysis, these results suggest the presence of neuroinflammation and significant neuroinflammatory transcriptional reprogramming in the mPFC during the early phase of PND.
qRT–PCR validation of RNA-seq results
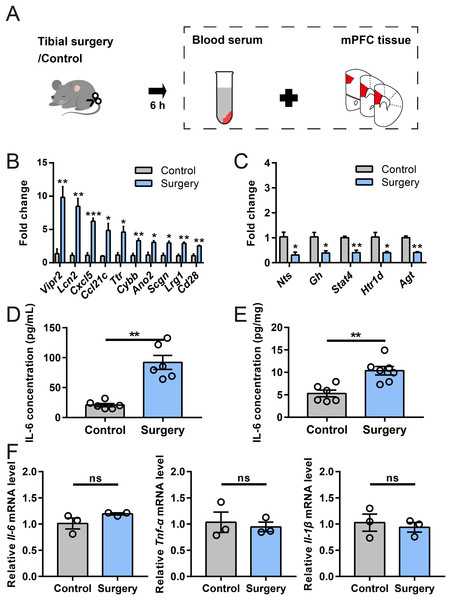
To validate the RNA-seq data, 15 DEGs of interest were selected for qRT–PCR analysis. The DEGs were both statistically robust and biologically relevant to neuroinflammatory pathways or cognitive function, aligning with our study’s focus (Table S3). Their expression analyzed by qRT–PCR exhibited changes similar to the RNA-seq data (Figs. 4B and 4C), with 10 genes (Vipr2, Lcn2, Cxcl5, Ccl21c,Ttr, Cybb, Ano2, Scgn, Lrg1, and Cd28) upregulated and five (Nts, Gh, Stat4, Htr1d, and Agt) downregulated. Among them, Vipr2, Lcn2, Ttr, Ano2, Scgn, Gh, Nts, and Agt have been implicated in neurological dysfunction and disease, and Lcn2, Cxcl5, Ccl21c, Cybb, and Cd28 play roles in immune responses. Other genes selected for qRT–PCR analysis included Lrg1, Stat4, and Htr1d, which are involved in signal transduction and neurotransmission. Importantly, all of these genes exhibited substantial regulation in both RNA-seq and qRT–PCR analyses.
Figure 4: Verification of DEGs and inflammatory cytokine levels.
(A) Cartoon depicting samples collection in another cohort at 6 h after surgery. (B and C) qRT–PCR validation of selected DEGs identified by RNA-seq analysis (n = 3 mice/group). (D and E) ELISA for IL-6 in serum and the mPFC, respectively (n = 6–7 mice/group). (F) Relative expression of Il-6, Tnf-α, and Il-1β in the mPFC (n = 3 mice/group). Data are represented as mean ± SEM, ***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant.Levels of inflammatory cytokines
To verify the immune response mediators, levels of representative proinflammatory cytokine were evaluated by ELISA and qRT–PCR (Fig. 4A). Notably, ELISA indicated that IL-6 level was significantly elevated in serum and mPFC 6 h post-surgery (Figs. 4D and 4E). However, no significant alternations were noticed in the mRNA levels of Il-6, Tnf-α and Il-1β in the mPFC after surgery (Fig. 4F). These results suggested that a distinct neuroinflammatory response was induced during early phase of PND.
Discussion
In this study, we demonstrated that cognitive function was impaired after tibial surgery in mice. Since the function of the mPFC region is dysregulated early after surgery (Sun et al., 2023), mPFC tissues were isolated 6 h post-surgery for RNA-seq analysis. Significant enrichment of the identified DEGs in immune-related processes and pathways was noticed. Additionally, we observed an increase in IL-6 level in the mPFC, whereas its mRNA level remained unchanged, indicating that a distinct neuroinflammatory response occurred in the mPFC.
Several DEGs have been implicated in various neurological diseases, and the majority were identified for the first time in PND. For example, Cxcl5, a prominently upregulated gene, is markedly elevated in response to brain injury and participates in neuroinflammatory responses and cognitive decline (Wang et al., 2016; Cao et al., 2023). Another upregulated gene, Ccl21, facilitates the recruitment of peripheral Th17 cells into the brain parenchyma, thereby exacerbating neuroinflammation and impairing cognitive abilities (Wang et al., 2022). Interestingly, elevated lipocalin-2 levels were observed in the mPFC, which contributes to neuroinflammation and cognitive decline in the hippocampus (Xiang et al., 2022). However, whether a similar mechanism exists remains unclear. Additionally, Nts, a down-regulated DEG, exerts neuroprotective effects in Alzheimer’s disease (Xiao et al., 2014).
Neuroinflammation is triggered after peripheral surgery, and is involved in developing PND. The mPFC is particularly susceptible to the effects of inflammation, and acute neuroinflammation can disrupt neural network and synaptic activity, resulting in significant cognitive deficits (Ji et al., 2020). In GO and KEGG pathway enrichment analyses, we observed significant enrichment in immune-related terms and pathways. First, DEGs were notably enriched in chemotaxis processes, including lymphocyte, monocyte, and neutrophil chemotaxis. Meanwhile, DEGs were enriched in the chemokine and chemokine receptor signaling pathways. Chemokine axes, such as CXCL5/CCR2 and CCL21/CCR7, can drive circulating leukocyte trafficking into the brain (Wang et al., 2016, 2022; Cao et al., 2023). Trafficking of immune cells, such as circulating CD8 T-cells and monocyte, into the brain impairs cognitive functions following surgery (Degos et al., 2013; Li et al., 2023). Second, DEGs were enriched in the Toll-like receptor signaling pathway, cellular response to IL-1, and TNFα signaling pathway. Toll-like receptors can sense danger and foreign molecules, thereby leading to the production of proinflammatory cytokines and impaired learning and memory functions (Lin et al., 2021; Squillace & Salvemini, 2022). Elevated levels of IL-1β induce an imbalance of excitation–inhibition in the mPFC (Mittli et al., 2023), and blocking IL-1β signaling mitigates neuroinflammatory response and improves the cognitive performance in PND (Cibelli et al., 2010; Barrientos et al., 2012). Similarly, neutralization of TNFα remarkably ameliorates surgery-induced neuroinflammation and cognitive deficits (Terrando et al., 2010, 2011). Third, IL-17 has emerged as an important player in several neurodegenerative diseases. Elevated levels of IL-17 trigger synaptic and circuitry impairments in early stage Alzheimer’s disease and autism (Choi et al., 2016; Brigas et al., 2021), and neutralization of IL-17 ameliorates sevoflurane-induced neurocognitive impairment (Zhang et al., 2022). Fourth, the complement cascade, which is recognized as an important player in synaptic pruning (Schafer et al., 2012), is activated after surgery and contributes to PND (Wu et al., 2023). Fifthly, although IFN-γ is necessary for social behavior (Filiano et al., 2016), elevated levels of IFN-γ prime microglial activation and the production of proinflammatory cytokines, ultimately impairing cognitive functions (Kann, Almouhanna & Chausse, 2022). Last but not least, uncontrolled synaptic pruning by astrocytes disrupts inter-neuronal connectivity, resulting in memory impairment (Lee et al., 2021). Astrocyte activation following peripheral surgery in the mPFC has been shown to compromise postoperative memory consolidation by promoting the phagocytosis of hippocampal neuronal axon terminals (Ma et al., 2024).
Our study has several limitations. First, while IL-6 protein and mRNA levels typically increase in parallel during inflammation (Gonçalves et al., 2008), we observed that the mRNA levels of Il-6, Tnf-α, and IL-1β remained unaltered, and IL-6 protein levels were elevated both in serum and mPFC. Neuroinflammation is a complex process, and the observed discrepancy in IL-6 protein and mRNA levels may result from several factors, including the choice of the observation timepoint, post-transcriptional regulatory mechanisms, transport across blood–brain barrier from circulating system, or a combination of these factors. It would be interesting to address these questions in future studies. Additionally, the protein levels of other proinflammatory cytokines including TNF-α and IL-1β remain to be determined. Second, considering cellular heterogeneity of the mPFC, single-cell RNA-seq and spatial transcriptomic techniques, such as multiplexed error-robust fluorescence in situ hybridization, would help advance our understanding of mPFC dysfunction after surgery and development of PND. Third, other brain regions may also be involved in cognitive decline after surgery. In fact, our previous studies identified 268 DEGs in the hippocampus after surgery (Xiang et al., 2019), and revealed that LCN2, one of the top elevated genes in hippocampus, mediates surgery-induced microglia activation and cognitive decline (Xiang et al., 2022). However, whether there is a common role of LCN2 in mPFC remains to be determined. Given the well-established roles of mPFC-hippocampal circuits in cognitive function, comparing transcriptomic responses in the mPFC and hippocampus at the circuit level would offer additional insights into PND. Finally, since we identified a series of novel DEGs, further gain- and loss-of-function studies are required to elucidate specific contributions of these genes to PND.
Conclusions
In summary, we identified a distinct and acute DEG signature in the mPFC after surgery, and revealed distinct features of the functional properties of the mPFC during the early phase of PND. Integrating these findings with further gain- and loss-of-function experiments would greatly help us to better understand of the development and pathogenesis of PND.