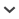In this review, we report on harvestmen and spiders feeding on fungi, fruits, and seeds. Fungivory in harvestmen is widespread, with most reports referring to tropical species in the family Sclerosomatidae, which consume mainly small forest mushrooms (families Marasmiaceae and Mycenaceae). In contrast, consumption of fungal material by spiders apparently occurs only if airborne spores trapped in the viscid threads of orb-webs (e.g., Araneidae and Tetragnathidae) are ingested along with old webs prior to the construction of new webs. Consumption of fruit pulp by harvestmen is also widespread, with several records of Leiobunum spp. (Sclerosomatidae) feeding on Rubus spp. berries and other lipid-poor fruits in the Holarctic region. In Neotropical forests, harvestmen in the families Cosmetidae and Gonyleptidae feed on lipid-poor pulp of fallen fruits. Among spiders, we document several cases of synanthropic species opportunistically feeding on fruit waste (e.g., pieces of banana, papaya, watermelon, or orange pulp) inside houses or disposed in yards. Only one case of a spider feeding on a wild fruit in the field was found in our search. Finally, we report several cases of harvestmen and spiders feeding on elaiosomes or arils (i.e., lipid-rich seed appendages). In conclusion, harvestmen consume mushrooms, fruit pulp, seeds, and seed appendages more frequently than spiders probably because they are “solid food feeders”, which means they can ingest solid tissues by biting off small pieces. In turn, spiders are “fluid feeders” and feed on vegetable matter most frequently in the form of fluids (e.g., nectar, stigmatic exudate, plant sap, and honey dew), rather than fungal or plant tissues.
1. INTRODUCTION
Harvestmen (Opiliones) and spiders (Araneae) are two speciose arachnid orders, which comprise together more than 56,000 species (Kury et al. 2021; World Spider Catalog 2022). Species in these orders exhibit an enormous diversity of lifestyles and foraging strategies in terrestrial ecosystems (Machado et al. 2007; Foelix 2011; Nyffeler & Birkhofer 2017). Harvestmen have been conventionally described as predators or omnivores and spiders as predators; both groups, however, depend largely on arthropods as food (Nentwig 1987; Wise 1993; Nyffeler et al. 1994; Acosta & Machado 2007). More recent studies expanded our understanding of the foraging behavior and diet of harvestmen and spiders, including observations of individuals feeding on small vertebrates (e.g., Castanho & Pinto-da-Rocha 2005; Benson & Chartier 2010; Oliveira et al. 2010; Nyffeler et al. 2017a; Nyffeler & Gibbons 2022a) and other unusual prey, such as gastropods (Nyffeler & Symondson 2001), earthworms (Nyffeler et al. 2017b), and eggs of invertebrates and vertebrates (Nyffeler & Gibbons 2022b).
In 2007, Acosta & Machado compiled a list of the various types of food items consumed by harvestmen, including reports of consumption of fruits, seeds, pollen, mushrooms, lichens, and algae. Almost ten years later, Nyffeler et al. (2016) published a review of plant-eating by spiders. These reviews bear witness that both harvestmen and spiders feed on a broad range of fungal and/or plant materials in addition to their usual arthropod prey. It must be said, however, that the number of reports on consumption of plant materials by harvestmen is very limited in number (Acosta & Machado 2007). For spiders, the number of reports of consumption of plant materials is considerably higher, but most of them refer to cases in which nectar and pollen are consumed (Nyffeler et al. 2016). In recent years, however, new evidence of vegetarian diet (i.e., plant and fungi consumption) in harvestmen and spiders has been published (e.g., Del-Claro et al. 2017; Eastburn 2017; Lietzenmayer & Wagner 2017; Nahas et al. 2017; Hyodo et al. 2018; Benhadi-Marín et al. 2019; Cathrine 2019; Pagoti et al. 2019; Suvák 2019; Hashimoto et al. 2020; Suzuki et al. 2021). The term “vegetarian” is currently used in relation to carnivores, which occasionally switch to plant and/or fungal food as an alternative to prey (e.g., Beckman & Hurd 2003; Wäckers & Fadamiro 2005; Meehan et al. 2009). In this paper, we define “vegetarian food” as diets of plant or fungal origin in addition to the arachnids’ consumption of prey.
Despite the increase in the records of fungal and plant materials in the diet of harvestmen and spiders, many questions concerning their vegetarian diet remain unanswered. In particular, it is still largely unexplored to what extent harvestmen and spiders can be engaged in fungivory and frugivory. To fill this gap, we conducted a survey on this topic with the purpose of searching for reports of fungivory and frugivory in species of these two arachnid orders. In this paper, the term “frugivory” is used in a broad sense, by including the consumption of fruit pulp, seeds, and seed appendages, such as elaiosomes (i.e., fleshy structures, usually rich in lipids and proteins, attached to the seeds of many plant species), and arils (i.e., a specialized outgrowth from a seed that partly or completely covers the seed) (Rico-Gray & Oliveira 2008). Based on the results of our review, we address four questions in our discussion: (1) How common is fungivory and frugivory among harvestmen and spiders? (2) Which taxa are engaged in fungivory and frugivory? (3) What are the nutritional benefits of fungivory and frugivory? (4) Can harvestmen and spiders be sustained on only fungal or fruit diets?
2. METHODS
2.1 Data collection.—We searched published reports on fungivory and frugivory in the Web of Science, Scopus, Google Search, Google Scholar, Google Books, and Google Pictures as well as ProQuest Dissertations and Theses, following the same search method as Nyffeler et al. (2017c). The combination of key words used in our search is presented in Appendix 1 (01. JoA 22-015 Supplemental Material.pdf) (see Supplemental Materials, online at https://doi.org/10.1636/JoA-S-22-015.s1). Additionally, we made a library search of books and scientific journals not included in the electronic databases. Social media sites (e.g., BugGuide, iNaturalist, Facebook, Flickr, Getty Images, Reddit, Yahoo, and YouTube) were also searched. Some of the bloggers who had posted photos and/or reports relevant to the review were contacted by us to obtain additional information. For harvestmen, a total of 96 records have been found, 41 of which refer to consumption of fungal material, 39 to consumption of fruit pulp, and 16 to consumption of seeds or seed appendages (Tables 1–3). By contrast, a total of 28 records relating to spiders have been found, 3 of which refer to consumption of fungal material, 12 to consumption of fruit pulp, and 13 to consumption of seeds or seed appendages (Tables 1–3).
2.2 Taxonomic comments.—Nomenclature of harvestman and spider taxa is based on the World Catalog of Opiliones ( http://wcolite.com/) and World Spider Catalog ( https://wsc.nmbe.ch/), respectively. Harvestmen depicted in Figs. 1A–H, 2A, 3A–D, and 4A–B were identified by the following researchers: James C. Cokendolpher (Phalangiidae and Sclerosomatidae), Adriano B. Kury (Cosmetidae, Gonyleptidae, Phalangiidae, and Sclerosomatidae), Ryosuke Kuwahara (Sclerosomatidae), Glauco Machado (Cosmetidae and Gonyleptidae), Jochen Martens (Gonyleptidae, Phalangiidae, and Sclerosomatidae), Miguel Medrano (Cosmetidae), and Jeffrey W. Shultz (Sclerosomatidae). Spiders were identified by the following researchers: Antonio D. Brescovit (Araneidae), G.B. Edwards and David Hill (Salticidae), and Robert Raven (Sparassidae). Fungi depicted in Figs. 1A–H and 2C–D were identified by the following researchers: Tim Baroni, Bart Buyck, and Kathie T. Hodge (Russulaceae), Dennis E. Desjardin and Serena Lee (Mycenaceae, Marasmiaceae, and Psathyrellaceae), Dennis E. Desjardin, Meike Piepenbring, and Steven L. Stephenson (a Myxomycetes slime mold), and Julieta Carranza (Pluteaceae). Fruits depicted in Figs. 3B, 3D, 3H, 4B–D, and Fig. S1 (01. JoA 22-015 Supplemental Material.pdf) (online at https://doi.org/10.1636/JoAS-22-015.s1) were identified by the following researchers: Maria do Carmo Amaral (Commelinaceae), Arik Hartmann (Chrysobalanaceae), Samantha Koehler (Combretaceae), Bruce Rottink (Fagaceae and Melanthiaceae), Renato Goldenberg (Melastomataceae), Gilbert Barrantes (Piperaceae), and Gustavo Shimizu (Primulaceae).
2.3 Chemical property information for fungi and fruits.—The following sources were used to obtain information on the chemical properties of the fungal material and fruit species consumed by harvestmen and spiders:
– Todd & Bretherick (1942); Bartnicki-Garcia (1968); Fogel & Trappe (1978); Barros et al. (2008); Chen et al. (2010) and Kalogeropoulos et al. (2013): Mushroom data for Cantharellaceae, Marasmiaceae, Russulaceae, and other Basidiomycota; spore data on Basidiomycota / fungi Incerta sedis.
– United States Department of Agriculture database (online at https://fdc.nal.usda.gov/index.html): Fruit/Yeast data for Adoxaceae, Bromeliaceae, Caricaceae, Cucurbitaceae, Fagaceae, Juglandaceae, Musaceae, Rosaceae, Rutaceae, and Saccharomycetaceae.
– Galetti et al. (2000): Fruit data for Euphorbiaceae, Meliaceae, Myristicaceae, and Myrtaceae.
– Aguiar et al. (2011) and Maldonado et al. (2020): Fruit data for Chrysobalanaceae.
– Udotong & Bassey (2015): Fruit data for Combretaceae.
– Messeder et al. (2021): Fruit data for Melastomataceae.
– Foster (1977): Fruit data for Primulaceae.
– Herbst (1986) and Bizerril & Raw (1997): Fruit data for Piperaceae.
– Lisci et al. (1996) and Fischer et al. (2008): Elaiosome data for Violaceae and other elaiosome-bearing plant taxa.
– Malacrida et al. (2011): Seed data for Caricaceae.
– Jordano (2000): Data for insects.
Table 1.
Reports of harvestmen and spiders feeding on mushrooms and other fungal material. In the column “Country”, the letter (F) indicates information gathered in the field and the letter (L) indicates information gathered in the laboratory. The column “Records” refers to the number of written reports (e.g., papers, theses, and books) or internet images of a given arachnid species feeding on a fungus species. Records followed by * were previously mentioned in the reviews of Acosta & Machado (2007) or Nyffeler et al. (2016).

Table 2.
Reports of harvestmen and spiders feeding on fruit pulp. In the column “Country”, the letter (F) indicates information gathered in the field and the letter (L) indicates information gathered in the laboratory. The column “Records” refers to the number of written reports (e.g., papers, theses, and books) or internet images of a given arachnid species feeding on the fruit pulp of a plant species. Records followed by * were previously mentioned in the reviews of Acosta & Machado (2007) or Nyffeler et al. (2016).

Table 2.
Continued.

2.4 Caloric value of fungi and fruits.—Caloric values for various types of fungi and fruits were taken for the most part from the United States Department of Agriculture database. Additional data were extracted from Fogel & Trappe (1978), Herbst (1986), Bizerril & Raw (1997). Caloric values for insects were adopted from Yhoung-Aree (2010). For some tropical fruits (i.e., Alchornea glandulosa, Ardisia sp., Cabralea canjerana, Eugenia stictosepala, Miconia sp., Terminalia capatta, and Virola bicuhyba), which have no caloric values available in the literature, the values were estimated using the Atwater system (Mayxard 1944). This was done by adding up the calories provided by the energy-containing nutrients (i.e., fat, protein, and carbohydrate). The Atwater system is based on average values of 9 kcal/g for fat, 4 kcal/g for protein, and 4 kcal/g for carbohydrate (Food and Nutrition Information Center (FNIC), online at https://www.nal.usda.gov/legacy/fnic/how-many-calories-are-one-gram-fat-carbohydrate-or-protein) and with knowledge of the fruits' chemical composition (Table 4). Calculations were accomplished yielding caloric values per 100 g of dry weight. Conversion to kcal/100 g of fresh weight was performed with knowledge of the fruits' water content (Table 4).
3. RESULTS
In the following, we report on cases of fungivory and frugivory in harvestmen and spiders. Consumption on seeds and seed appendages (elaiosomes and arils) was included in the subsection on frugivory because seeds are a component of the fruits. A total of 96 records of fungivory/frugivory referring to harvestmen are reviewed in the present paper (Tables 1–3), 83% of which were not mentioned in the review of Acosta & Machado (2007). Twenty-eight records of fungivory/frugivory referring to spiders are presented (Tables 1–3), 86% of which were not found in the review of Nyffeler et al. (2016).
3.1 Fungivory.—In general, arachnids can consume mushroom tissue and sap, fungal spores, or yeast (Figs. 1–2).
3.1.1 Feeding on mushroom pileus or sap: Fungivory by harvestmen has been known for a long time (e.g., Šilhavý 1942; Bristowe 1949; Goodnight & Goodnight 1960). Consumption of fungal material has been witnessed on five continents (Europe, North America, Central America, South America, and Asia). Most of the records (61%) refer to tropical harvestmen in the family Sclerosomatidae (predominantly subfamily Gagrellinae) (Table 1). The remaining cases relate to the families Cladonychiidae, Cosmetidae, Gonyleptidae, Nomoclastidae, and Phalangiidae; in some cases, family identity is unknown (Table 1). Under field conditions, 64% of the reports on fungivory included small forest mushrooms belonging to the families Marasmiaceae and Mycenaceae (dominated by Mycena spp., Figs. 1A–G), 17% of the reports included mushrooms belonging to the families Cantharellaceae, Plutaceae, Psathyrellaceae (Fig. 2A), and Russulaceae (Fig. 1H), and in 19% of the reports the identity of the family was unknown (Table 1).
At least some of the fungi from the families Marasmiaceae and Mycenaceae are bioluminescent in the dark (Fig. 2B). Harvestmen are known to forage predominantly during the night hours (Acosta & Machado 2007) and appear to be attracted by the light emission of bioluminescent mushrooms (Fig. 2B; Luping et al. 1978; Waldenmaier 2016). In fact, it has been experimentally demonstrated that the New Zealand harvestmen Forsteropsalis tumida (Forster, 1944) (Neopilionidae) and Hendea myersi (Phillipps & Grimmett, 1932) (Triaenonychidae) are attracted to the light emitted by bioluminescent glow-worms (Meyer-Rochow & Liddle 1988, 2001).
Table 3.
Reports of harvestmen and spiders feeding on seeds (S) or seed appendages (E = elaiosomes, A = arils). In the column “Country”, the letter (F) indicates information gathered in the field and the letter (L) indicates information gathered in the laboratory (including greenhouses). The column “Records” refers to the number of written reports (e.g., papers, theses, and books) or internet images of a given arachnid species feeding on seeds or seed appendages of a plant species. Non-identified harvestmen sp.2†, sp.3†, and sp.8† were described by Ohkawara & Higashi (1994) and Ohkawara et al. (1996, 1997) as spiders, but a picture sent to us by Ohkawara clearly shows that the animals in question were harvestmen. Records followed by * were previously mentioned in the reviews of Acosta & Machado (2007) or Nyffeler et al. (2016).

There are reports of fungivory among parental males of two species of the genus Quindina Roewer, 1915 (Nomoclastidae) (Rodríguez & Guerrero 1976; Mora 1990; Requena & Machado 2015; Rojas et al. 2019). In this genus, males build mud nests on fallen tree trunks that are used by females as oviposition sites. The nests are usually infested by fungi, which can kill the eggs (Mora 1990; Quesada-Hidalgo et al. 2019; Rojas et al. 2019). Males feed on the fungi, keeping the nest clean and the eggs alive (Figs. 2C–D).
Figure 1.
Harvestmen feeding on mushrooms. A. Unidentified Gagrellinae (Sclerosomatidae) feeding on the mushroom Marasmius sp. (Marasmiaceae) in Singapore (Photo by Melvin Yeo). B. Gagrella spinacantha Roewer, 1954 (Sclerosomatidae, Gagrellinae) feeding on the mushroom Marasmius sp. (Marasmiaceae) in Singapore (Photo by Melvin Yeo). C. Unidentified Gagrellinae (Sclerosomatidae) feeding on the mushroom Crinipellis sp. (Marasmiaceae) in Singapore (Photo by Melvin Yeo). D. Marthana niveata (Roewer, 1955) (Sclerosomatidae, Gagrellinae) feeding on the mushroom Marasmius sp. (Marasmiaceae) in Malaysia (Photo by Hock Ping Guek). E. Pseudogagrella sp. (Sclerosomatidae, Gagrellinae) feeding on a mushroom (Mycenaceae) in Malaysia (Photo by Hock Ping Guek). F. Gagrellula ferruginea (Loman, 1902) (Sclerosomatidae, Gagrellinae) in the process of tearing off the pileus (≈0.5 cm in diameter) of the mushroom Mycena sp. (Mycenaceae) in Japan (Photo by Ryosuke Kuwahara). G. Unidentified Gagrellinae (Sclerosomatidae) feeding on the mushroom Mycena sp. (Mycenaceae) in Singapore (Photo by Melvin Yeo). H. Leiobunum sp. (Sclerosomatidae, Leiobuninae) feeding on the mushroom Russula sp. (Russulaceae) in Hart County, Kentucky, USA (Photo by Chuck Rosenberger).

Figure 2.
Further cases of harvestmen feeding on mushrooms. A. Unidentified Cosmetidae feeding on the mushroom Coprinellus sp. (Psathyrellaceae) in Tambopata area, Peru (Photo by Rhett Butler). B. Bioluminescent mushrooms growing on the forest floor in Singapore emit a greenish light at night. Unidentified harvestmen were often seen feeding on the pileus of such bioluminescent mushrooms (Photo by Nicky Bay). C. Male of Quindina albomarginis (Chamberlin, 1925) (Nomoclastidae) inside his mud nest built on a fallen log in Panama (the dorsum and hind legs are marked with colored ink). Note that, by feeding on the growing fungi (a Myxomycetes slime mold), the parental male keeps the nest floor and the eggs cleaner when compared to the areas outside the nest (Photo by Gustavo S. Requena). D. Male of Quindina limbata (Roewer, 1943) (Nomoclastidae) inside his mud nest built on a fallen log in Costa Rica. Again, the parental male keeps the nest and the eggs clean by feeding on the fungi that grow on the fallen trunk (Photo by Andrés Rojas).

Spiders are frequently found resting near or on mushrooms (see the large number of examples uploaded on Google Pictures using the key words “spider + mushroom”), but they have never been reported feeding on the pileus or stipe of mushrooms. This was confirmed by a nocturnal infrared video surveillance study conducted in the Atlantic Rainforest near São Paulo, southeastern Brazil, in two consecutive years (Waldenmaier 2016). According to this surveillance study, ground spiders from the families Gnaphosidae and Lycosidae frequently visited the area of Mycena luxaeterna, staying there for up to 30 min, but always without feeding on the mushrooms (Waldenmaier 2016). The only mentions of naturally occurring fungivory in spiders are cases of ingestion of fungal spores by orb-weavers (see next subsection).
3.1.2 Feeding on fungal spores and yeast: Currently, reports of consumption of fungal spores by harvestmen are lacking (see Acosta & Machado 2007; Lundgren 2009). Spiders, on the other hand, get access to fungal spores as a food source if airborne fungal spores blown by wind into their webs get stuck to the viscid threads (Smith & Mommsen 1984; Linskens et al. 1993; Del Fiol et al. 2007). The spores are ingested along with the old web prior to the construction of a new web, as has been shown in laboratory experiments with the orb-weaver Araneus diadematus Clerck, 1757 (Araneidae) (Smith 1984; Smith & Mommsen 1984). The digestive fluid of spiders contains the enzyme chitinase needed to dissolve and digest the chitinous spore cell wall, which explains why spiders can digest fungal spores (Mommsen 1978, 1980; Smith & Mommsen 1984; Nyffeler et al. 2016). Spores from many different fungal families (e.g., Botryosphaeriaceae, Davidiellaceae, Helotiaceae, Massarinaceae, Microascaceae, Nectriaceae, Phragmidiaceae, Pleosporaceae, Trichocomaceae, Trichosphaeriaceae, and Venturiaceae) are blown by wind into spider webs (Smith & Mommsen 1984; Linskens et al. 1993; Bera et al. 2002; Del Fiol et al. 2007; Quamar & Chauhan 2011; Nyffeler et al. 2016). The spores trapped in spider webs belong to the most common fungal genera one would expect to find in the air (Nyffeler et al. 2016). In Italy, Del Fiol et al. (2007) found ≈17,000 fungal spores trapped in six orb-webs of the spider A. diadematus (sampled from summer of one year to spring of the next year), leading to the conclusion that fungal spores might be a supplementary non-prey food of some nutritional importance (but see subsection “What are the nutritional benefits of fungivory and frugivory?” in Discussion). Besides fungal spore consumption, linyphiid spiders consumed yeast material in laboratory feeding trials (Sunderland et al. 1996). Consumption of yeast under laboratory conditions was observed in harvestmen as well (Todd 1949; Edgar 1971).
3.2 Frugivory.—This type of feeding behavior refers to the consumption of fruit pulp, seeds, and seed appendages, such as elaiosomes and arils (Figs. 3–4). While frugivory by harvestmen has been known for a long time, frugivory by spiders is a new area of research (see also Nyffeler et al. 2016).
Figure 3.
Harvestmen and spiders feeding on fruits. A. The harvestman Eupoecilaema magnum Roewer, 1933 (Cosmetidae) feeding on a ripe Piper sp. fruit directly on the shrub in Costa Rica (Photo by Gilbert Barrantes). B. Three individuals of the harvestman Gryne dimorpha Mello-Leitão 1928 (Cosmetidae) and one individual of the harvestman Magnispina neptunus Mendes, 2011 (Gonyleptidae) feeding on a fallen fruit of Terminalia catappa (Combretaceae) in Northeastern Brazil (Photo by Rodrigo H. Willemart). C. The harvestman Gagrellula ferruginea (Loman, 1902) (Sclerosomatidae) feeding on an unidentified wild fruit in Japan (Photo by Ryosuke Kuwahara). D. The harvestman Phalangium opilio Linnaeus, 1761 (Phalangiidae) feeding on an oak acorn fruit of Quercus sp. (Fagaceae) in USA (Photo by Darlene Watson). E. The orb-weaving spider Alpaida leucogramma (White, 1841) (Araneidae) feeding on a piece of a papaya fruit waste (Caricaceae) thrown into a yard in Cayenne, French Guiana (Photo by Sean McCann). F. The spider Philodromus sp. (Philodromidae) feeding on a speck of papaya pulp left on the kitchen counter in a residence in Campbell River, British Columbia, Canada (Photo by Susannah Anderson). G. The yellow sac spider Cheiracanthium sp. (Cheiracanthiida) feeding on a slice of blood orange (Rutaceae) that had been left on a kitchen counter cutting board in Ann Arbor, Michigan, USA (Photo by Isa S. Betancourt). H. The yellow sac spider Cheiracanthium inclusum (Cheiracanthiidae) feeding on an overripe cocoplum fruit of Chrysobalanus icaco (Chrysobalanaceae) in the Everglades, USA (Photo by Arik Hartmann).

Figure 4.
Seeds bearing elaiosomes and arils that are consumed by predatory arthropods, including harvestmen and spiders. A. Harvestman – presumably an immature of Nelima paessleri (Roewer, 1910) (Sclerosomatidae) – feeding on elaiosomes of Trillium ovatum (Melanthiaceae) in a forested area in Oregon, USA (Photo by Bruce Rottink). B. Open capsule of the fruit of Dichorisandra paranaensis (Commelinaceae) showing the seeds surrounded by a white aril. The aril of one of these seeds is chewed by the harvestman Serracutisoma proximum (Mello-Leitão 1922) (Gonyleptidae) in southeastern Brazil (Photo by Bruno A. Buzatto). C. Seed of Cabralea canjerana (Meliaceae) fallen on the leaf litter in the Brazilian Atlantic forest. Seeds of this species are surrounded by a lipid-rich aril that is consumed by several ant species (such as the Pheidole sp. depicted in the photo) and by the harvestman Neosadocus bufo (Photo by Marco Aurélio Pizo). D. Seed of Virola bicuhyba (Myristicaceae) fallen on the leaf litter in the Brazilian Atlantic forest. Seeds of this species are also surrounded by lipid-rich aril that is consumed by ants (such as the Pheidole sp. depicted in the photo) and by the harvestman N. bufo (Photo by Marco Aurélio Pizo). E. The spider Parasteatoda sp. (Theridiidae) feeding on elaiosome-bearing seed of Costus dubius (Costaceae) in a greenhouse in Slovakia (Photo by Martin Suvák). F. The spider Parasteatoda sp. (Theridiidae) feeding on elaiosome-bearing seed of Hepatica nobilis (Ranunculaceae) during a greenhouse experiment (Photo by Martin Suvák).

3.2.1 Feeding on fruit pulp: Consumption of fruit pulp by harvestmen has been reported from six continents (Europe, Asia, Oceania, North America, Central America, and South America), and was observed in seven families (Cosmetidae, Globipedidae, Gonyleptidae, Nemastomatidae, Neopilionidae, Phalangiidae, and Sclerosomatidae; Table 2). In the Holarctic region, harvestmen in the genus Leiobunum (Sclerosomatidae) were repeatedly seen feeding on Rubus spp. (Rosaceae) berries (Edgar 1971; Halaj & Cady 2000; Wijnhofen 2011; Shardlow 2013; see also Bugguide, online at https://bugguide.net/node/view/1541187). In Neotropical forests, harvestmen from the families Cosmetidae and Gonyleptidae fed on the pulp of fallen fruits (Gnaspini 1996; Machado & Pizo 2000; Acosta & Machado 2007; Pagoti et al. 2019; Fig. 3B; Fig. S1C (01. JoA 22-015 Supplemental Material.pdf)). In two cases, species from the families Cosmetidae and Gonyleptidae were also observed feeding on ripe fruits directly on trees and shrubs (Fig. 3A; Fig. S1B (01. JoA 22-015 Supplemental Material.pdf)).
In the laboratory, there are also records of harvestmen consuming fruit pulp (Table 2). In the Holarctic region, Lacinius dentiger (Koch, 1847) (Phalangiidae) accepted apples and pears (Mitov 1988), and several species of neopilionids also accepted apples (Table 2). In the Neotropical region, the gonyleptids Heteromitobates discolor (Sørensen, 1884) and Mischonyx squalidus Bertkau, 1880 accepted banana (Costa et al. 2016; Segovia et al. 2019; Dias et al. 2020), Opisthoplatus prospicuus (Holmberg, 1876) accepted cucumber (Fernandes et al. 2017), Discocyrtanus pertenuis (Mello-Leitão, 1935) accepted pear ( Fig. S1A (01. JoA 22-015 Supplemental Material.pdf)), and Acanthopachylus aculeatus (Kirby, 1819) and Promitobates ornatus (Mello-Leitão, 1922) accepted papaya (Capocasale & Bruno-Trezza 1964; Willemart 2001).
Table 4.
Major chemical properties and caloric value (kcal/100 g) for various taxa of fungi and fruits used as food by arachnids (see Tables 1–3). Abbreviations: A = Araneae (spiders); O = Opiliones (harvestmen); E (superscript) = information obtained in experiments; FW = fresh weight; DW = dry weight; # = combined value for proteins + amino acids; * = values roughly estimated using the Atwater system (see Methods subsection 2.4); ** = chemical composition of the fleshy aril; NA = non-available information.

More recently, several cases of free-living spiders feeding on fruits have been witnessed (Table 2). In central Scotland, Cathrine (2019) witnessed on several occasions males of Amaurobius similis (Blackwall, 1861) (Amaurobiidae) feeding on remains of banana fruit pulp (Musa sp.; Musaceae) left on a kitchen table. This unusual feeding behavior in Amaurobius was witnessed over several years in two residencies located about 11 km apart (Cathrine 2019). There are similar reports from North America and South America of free-living spiders feeding on fruit remains (e.g., pieces of papaya, watermelon, or orange pulp) left over on kitchen counters or disposed in yards (Table 2; Figs. 3E–G).
Under field conditions, an American yellow sac spider, Cheiracanthium inclusum Hentz, 1847 (Cheiracanthiidae), was observed feeding on an overripe cocoplum fruit (Chrysobalanus icaco; Chrysobalanaceae) in the Everglades National Park, Florida, USA (Fig. 3H). Chrysobalanus icaco is a common native shrub in the Everglades ecosystem. Continued observation showed the spider manipulating the pulp of the fruit with its chelicerae (see https://figshare.com/articles/media/American_yellow_sac_spider_Cheiracanthium_inclusum_Cheiracanthiidae_feeding_on_an_overripe_cocoplum_fruit_Chrysobalanus_icaco_Chrysobalanaceae_/19349174 Accessed March 10th 2022). It is unknown if the spider employed digestive enzymes to liquefy the flesh of the fruit, or if the ripened flesh was of appropriate consistency to be imbibed by the spider.
3.2.2 Feeding on seeds and seed appendages: We found 16 records of harvestmen feeding on seeds or seed appendages (Table 3). These reports refer in most cases (≈81%) to the consumption of elaiosomes and arils. In the Holarctic region, harvestmen were observed consuming the elaiosomes of plants in the families Aristolochiaceae, Berberidaceae, Liliaceae, Melanthiaceae, Papaveraceae, and Violaceae (Table 3; Fig. 4A). In the Neotropical region, there is a record of the gonyleptid Serracutisoma proximum (Mello-Leitão, 1922) feeding on the aril of a Commelinaceae (Fig. 4B). Moreover, the gonyleptid Neosadocus bufo (Mello-Leitão, 1923) has been regularly observed feeding on the fleshy arils of the capsular fruits of Alchornea glandulosa, Cabralea canjerana, and Virola bicuhyba (Machado & Pizo 2000). The arils of these three plant species become exposed to ground dwelling arthropods, such as ants and harvestmen, after the capsules open and the seeds fall on the forest floor (Figs. 4C–D).
Several records of spiders seen feeding on seeds or seed appendages were reported in recent years (Table 3). Berland (1933) was perhaps the first to report an araneid orb-weaving spider, Neoscona adianta (Walckenaer, 1802), sucking a grass seed (Poaceae). A similar incident – also concerning an araneid orb-weaving spider feeding on a plant seed – was witnessed recently in a residential area of the city of Sandnes, Norway (Olav Berge Aamodt, pers. comm.). Moreover, a huntsman spider, Heteropoda jugulans (L. Koch, 1876) (Sparassidae), was filmed in the kitchen of a residence in Brisbane, Queensland, Australia, feeding on a papaya seed that had been removed from a cutting board (Yahoo News Australia 2020). Finally, in a greenhouse in Slovakia, the comb-footed spider (Parasteatoda sp., Theridiidae) was seen feeding on elaiosomes of the elaiosome-bearing seeds of Costus dubius (Costaceae) trapped in its web (Fig. 4E; Suvák 2019). To learn more about the capability of spiders to use elaiosomes as a potential food source, Suvák (2019) conducted a greenhouse experiment by throwing elaiosome-bearing seeds of various plant taxa into webs of the spiders Parasteatoda sp. and Uloborus plumipes Lucas, 1846 (Uloboridae). This experiment revealed that both species fed readily on the elaiosomes (Table 3; Fig. 4F).
4. DISCUSSION
4.1 How common is fungivory and frugivory in harvestmen and spiders?—Consumption of fungal material by harvestmen has been reported more frequently in warmer areas, with >70% of all reports originating from tropical locations, especially Southeast Asia (Table 1). In contrast to harvestmen, consumption of fungal material by spiders seems to be less frequent (Table 1). However, consumption of fungal spores by spiders does occur all over the world and is not limited to a specific region (e.g., Smith 1984; Smith & Mommsen 1984; Bera et al. 2002; Del Fiol et al. 2007; Walter et al. 2019).
Feeding on fruit pulp, seeds, and seed appendages (i.e., elaiosomes and arils) by harvestmen was reported from areas of temperate and tropical climates (Tables 2–3). Feeding on fruit pulp and seeds by spiders was also reported from different parts of the world, but this appears to be largely limited to cases in which synanthropic species opportunistically feed on small pieces of fruit waste encountered inside houses or disposed in yards (Tables 2–3). Consumption of seed appendages, in turn, has been reported in a greenhouse experiment with two spider species (Table 3), and it is not possible to know how frequent this feeding habit is under natural field conditions.
4.2 Which taxa are engaged in fungivory and frugivory?—In the survey by Acosta & Machado (2007), information on the diet of harvestmen is available for 13 families (≈24% of all living families) and vegetarian feeding habits (ie., consumption of fungi, fruits, seeds, and seed appendages) have been documented for four families: Cladonychiidae, Gonyleptidae, Phalangiidae, and Sclerosomatidae. In the present review, we expand the number of harvestman families with records of vegetarian feeding, which now also includes Cosmetidae, Globipedidae, Nemastomatidae, Neopilionidae, and Nomoclastidae (Tables 1–3). Most of the documented feeding events summarized here refer to the Sclerosomatidae and Gonyleptidae, which are the largest harvestman families (Machado et al. 2007; Kury et al. 2021). Considering that 54 living families exist in the order Opiliones, it follows that little is known whether species of 45 families also engage in vegetarian feeding. There are two non-mutually exclusive explanations for this gap in our knowledge. First, some harvestman taxa apparently are exclusively carnivorous feeders (e.g., families Ischyropsalididae and Trogulidae; see Nyffeler & Symondson 2001 and Acosta & Machado 2007). Second, the feeding habits of most harvestman taxa are still unexplored (Powell et al. 2021). However, based on anecdotal evidence accumulated so far, we anticipate that consumption of fungi, fruit pulp, seeds, and seed appendages is widespread in many harvestman families, especially those that inhabit tropical forests, where the availability of these food items is probably high over most parts of the year, including the dry and cold season, when arthropod prey are scarcer (Wolda 1988).
For spiders, the situation is somewhat different. So far, fungivory has been documented under natural conditions exclusively for ecribellate orb-weaving spiders (family Araneidae), which digest airborne fungal spores during the recycling process of old webs prior to the construction of new webs (Smith & Mommsen 1984; Table 1). It is possible that web-building spiders other than orb-weavers also occasionally feed on fungal material trapped in their webs, as the laboratory feeding trials of Sunderland et al. (1996) suggest. Frugivory, in turn, seems to occur predominantly among synanthropic spiders from different families that opportunistically encounter small pieces of fruit waste mostly inside houses (Table 2). Frugivory by spiders under natural field conditions is documented here for the first time, including one species of the genus Cheiracanthium (Table 2). This result could be biased by the synanthropic spiders being probably more often observed by humans, when compared with the wild ones. Finally, the few cases of spiders feeding on seed appendages reported here (Table 3) appear to be uncommon occurrences, restricted to greenhouse conditions. In the last three decades, a large number of quantitative prey analyses on spiders have been conducted without detecting them feeding on fruit pulp, seeds, or seed appendages (see Nentwig 1987; Wise 1993; Nyffeler 1999; Pekár & Toft 2015). Thus, we suggest that, although frugivory by spiders may have been overlooked by arachnologists in the past, this type of feeding habit is probably rare in this order.
4.3 What are the nutritional benefits of fungivory and frugivory?—As pointed out in the Results, harvestmen and spiders use fungal material in different ways. Harvestmen that feed on fungal tissues (especially mushrooms) are provided with an abundant supply of water, carbohydrates, protein, minerals, and vitamins (Lundgren 2009). In particular, the high protein content (usually ≈20–40% on a dry weight basis; Table 4) of mushroom tissues is remarkable, but compared to insect prey, mushroom tissues have a low caloric value (≈30–50 kcal/100 g of fresh weight; Table 4). Furthermore, some mushrooms (e.g., Russula spp.) contain toxic substances (Matsuura et al. 2016), but nothing is known of harmful effects on harvestmen caused by the consumption of toxic mushrooms.
The situation is very different regarding the consumption of fungal spores by spiders. Spores have a high caloric value (≈300 kcal/100 g of fresh weight; Table 4) accompanied by a low water content (≈15%). Contrary to fungal tissues, the protein content of the spores is rather low (≈10–16% on a dry weight basis; Table 4), which renders them as food of very low or no nutritive value to spiders (Smith & Mommsen 1984; Lundgren 2009; Parish et al. 2020). Smith & Mommsen (1984) have shown in laboratory feeding trials that second instar spiderlings of the orb-weaving spider Araneus diadematus which had access to fungal spores as a potential food source did not differ significantly in their chance of survival from a starved control group.
The suitability of fungal spores as a diet might be further compromised by the presence of noxious secondary compounds (Smith & Mommsen 1984; Lundgren 2009). An experiment in which a mixed diet of plant pollen and yeast (family Saccharomycetaceae) was offered to early instar spiderlings of the sheet-web spider Tenuiphantes tenuis (Blackwall, 1852) (Linyphiidae) confirmed the hypothesis that the ingestion of fungi has a detrimental effect. Spiderlings feeding on the pollen-yeast diet showed a 50% decrease in survival time compared to a group of starved spiderlings (Sunderland et al. 1996). As a final remark, we stress that it is currently unknown whether the consumption of fungal spores plays a role in the dynamics of araneopathogenic fungi whose spores are infective propagules (Durkin et al. 2021). This would require spores to come into contact with, and adhere to, the host cuticle during consumption, as infection from inside the gut is unlikely (as far as we know).
Fruits can be classified into high-quality and low-quality according to their caloric value (Johnson et al. 1985). High-quality fruits usually have a water content of ≈40–60%, are rich in lipids (≈60–70% on a dry weight basis) and have a high caloric value (≈250–400 kcal/100 g of fresh weight; Table 4). In turn, fruits considered to be of low quality, are less nutritious (the pulp containing < 10% lipid on a dry weight basis; Table 4) with a watery flesh (≈70–90% moisture; Table 4). Such fruits have a high content of carbohydrates, some of which are in the form of sugars (i.e., fructose, glucose, and sucrose). Fruits of this type (e.g., apple, banana, berries, cocoplum, cucumber, elderberry, orange, papaya, pear, plum, pineapple, and watermelon) are a poor source of energy (≈15–70 kcal/100 g of fresh weight; Table 4). Most cases in which harvestmen and spiders have been reported to feed on fruit pulp refer to low-quality fruits (Table 4). This pattern raises the question whether feeding on fruits of low caloric value is of any nutritional benefit. Cathrine (2019), who observed spiders feeding on banana waste, commented “Whether this is for sustenance or moisture is unclear. . .”. Several researchers conducted food choice experiments under laboratory conditions and these experiments may provide an answer to the question raised above.
Schaus et al. (2013) carried out a feeding trial in which the Neotropical harvestman Erginulus clavotibialis (Pickard-Cambridge, 1905) (Cosmetidae) was given a choice between fresh pineapple (i.e., low-quality fruit) and live invertebrate prey. This harvestman demonstrated a distinct preference for fruit over invertebrate prey, clearly suggesting that feeding on fresh fruit was nutritionally beneficial to the individuals even though the offered fruit was of the low-quality type. Furthermore, Schaus et al. (2013) showed that individuals feeding on fruits were more active compared to starved individuals or individuals that fed on live invertebrate prey.
Analogous food choice experiments were conducted with spiders in the families Salticidae, Thomisidae, and Anyphaenidae (Vogelei & Greissl 1989; Pollard et al. 1995; Jackson et al. 2001; Taylor & Bradley 2009; Pfannenstiel & Patt 2012). In these experiments, the spiders were permitted to choose between a sucrose solution and distilled water. In all experiments the spiders spent more time drinking from the sucrose solution compared to water, showing that the spiders fed on sugar solutions to obtain nutrients and not just moisture. In these experiments, it has also been shown that consumption of sucrose solution increased spider survival significantly compared to consumption of water only. If we extrapolate these experimental findings to the situation of harvestmen and spiders feeding on low-quality fruits under natural field conditions, we conclude that they feed on fruits probably to obtain nutrients in addition to moisture.
Not only do harvestmen and spiders feed on fruit pulp, but they also feed on the fruits' most inner parts, the seeds and, more frequently, their appendages. In fact, most cases in which harvestmen and spiders were documented feeding on seeds refer to the consumption of elaiosomes or arils (Table 3). The nutritional quality of elaiosomes and arils as food resembles that of insects as regards their fatty acid composition (Hughes et al. 1994). Moreover, the lipid content of elaiosomes and arils is usually very high (Rico-Gray & Oliveira 2008; see also Table 4). For instance, the arils of Alchornea glandulosa, Cabralea canjerana, and Virola bicuhyba contain a much higher percentage of lipids (62–71%) when compared to the average value of various insect prey, which is around 17% (see Table 4). Various insect groups, particularly ants, are known to feed on elaiosomes and arils (Lundgren 2009; see also Figs. 4C–D). Here we showed that elaiosomes and arils are also consumed by arachnids. Whereas consumption of elaiosomes and arils by harvestmen is probably widespread, it has been reported for only a few spider genera in two families, always under artificial conditions (Table 3).
4.4 Can harvestmen and spiders be sustained on only fungal or fruit diets?—On Fukue Island and in other regions of Japan, some harvestman species (eg., Gagrellula ferruginea) are often seen feeding on the pileus of luminescent mushrooms (Mycena luxurius and Mycena spp.) growing on the forest floor (Fig. 1F; Uyemura 1935; http://fukuejima.la.coocan.jp/kinoko/shiino-tomoshibi-take.html; Ryosuke Kuwahara, pers. comm.). Likewise, harvestmen were reported eating on luminescent mushrooms of the family Mycenaceae growing on the ground of lowland rain forests in Malaysia (Luping et al. 1978) and Singapore (Melvin Yeo, pers. comm.). Furthermore, in the Brazilian Atlantic Forest, unidentified harvestmen were observed feeding on pileus tissue of the mushroom Mycena luxaeterna, which emits intense light during the night (Waldenmaier 2016). In all these examples, harvestmen were reported to feed heavily on mushrooms, indicating that fungivory may play an important role in the diet of certain species.
The genus Quindina is an interesting model system to study fungivory in harvestmen. During the period of paternal care, which may last several months, males remain most of the time inside their mud nests protecting their eggs and waiting for the visit of ovigerous females (Mora 1990; Quesada-Hidalgo et al. 2019). Although paternal care constrains foraging activity of the caring males, their body condition does not decrease over time (Requena & Machado 2015). This finding contrasts with another harvestman species with exclusive paternal care, Iporangaia pustulosa Mello-Leitão, 1935 (Gonyleptidae), in which the body condition of caring males deteriorates over time (Requena et al. 2012). One possible explanation for this difference is that caring males of Quindina regularly feed on the fungi that grow inside and around their nests (Figs. 2C–D) whereas caring males of I. pustulosa most likely do not have access to this food source (Requena & Machado 2015). In the future, it could be tested whether caring males of Quindina that are somehow prevented from feeding on fungi are able to sustain their body condition over the period of parental care.
We are not aware of any experimental study in which harvestmen were fed with exclusively mushrooms and we therefore do not know how they would respond under such unbalanced nutritional conditions. For spiders, this question has been tested by means of laboratory feeding trials. Spiderlings fed a diet consisting of only fungal spores (Cladosporium sp.) did not live longer than starving spiderlings, suggesting that the spores were of no nutritional value. Similar experimental findings have been reported for other arthropods. If a diet consisting of only fungal spores (Cladosporium sp. and two other fungal species) was offered to honeybees, this type of food was rejected (Parish et al. 2020). In another study, consumption of fungal spores by honeybees was shown to be detrimental, suggesting that the spores contain some type of toxic secondary compound (Schmidt et al. 1987). Fungal spores appear to be low-quality food probably because they have rigid protective walls – made up of complex three-dimensional network of polysaccharides – that make it difficult to access the nutritious inner content (Lundgren 2009; Noothalapati et al. 2016). In addition, fungal spores have low contents of protein and moisture compared to mushrooms. Thus, fungivory appears to be of little relevance to spiders from a nutritional point of view (see subsection “4.3 Nutritional benefits of fungivory” above).
Regarding frugivory, studies conducted with Neotropical harvestmen from the families Cosmetidae and Gonyleptidae suggest that opportunistic frugivory may be important whenever fruits, seeds, and seed appendages are abundant (Machado & Pizo 2000; Schaus et al. 2013; Pagoti et al. 2019). Harvestmen are considered to be diet generalists and feeding on mixed diets of plants and invertebrate prey is in several ways advantageous because: (1) it broadens the trophic niche, (2) it attenuates competition for food with other predatory arthropods, (3) it allows to switch increasingly to plant resources at times when invertebrate prey becomes scarce, and (4) it dilutes adverse effects of toxic compounds contained in some food sources (see Coll & Guershon 2002). However, when harvestmen were fed an only plum diet (Prunus domestica), they lost weight compared to harvestmen fed a diet containing arthropod prey (Hvam & Toft 2008). This indicates that, although harvestmen are rather generalist feeders, they may be unable to survive on a diet composed exclusively of low-quality fruits, such as plum (Hvam & Toft 2008; Table 4). There is a parallel to this in bird trophic biology. Experiments in which certain bird species were offered only fruit diets, likewise have shown that individuals lost weight, and this was attributed to the extreme lipid and protein deficiency of a low-quality fruit diet (Bairlein 1996; Jordano 2000).
For spiders, no experiment like the one reported above for harvestmen has been performed so far. However, it can be said that, contrary to harvestmen, consumption of fruit pulp and seed appendages by spiders under natural conditions is apparently uncommon, so that this issue seems to be of little relevance to this arachnid group. Bagheera kiplingi Peckham & Peckham, 1896 (Salticidae) is the only spider species known so far to exhibit a predominantly vegetarian lifestyle (Meehan et al. 2009). But even this species, which feeds to a large extent on the detachable leaf tips of Acacia trees (i.e., Beltian bodies) and nectar, individuals perish after one to several weeks and always before molting to the next instar if kept strictly on a plant-based diet (Nyffeler et al. 2016).
5. CONCLUDING REMARKS
Like scorpions, harvestmen are “solid food feeders”, which means that they can consume solid tissue of mushrooms, fruit pulp, seeds, or seed appendages as a food source by biting off small pieces. Another possibility could be that harvestman would need alternative sources of food as they are typically bad at capturing prey compared to other arachnids. The fact that 3.4 times as many records of harvestmen feeding on mushrooms and fruits could be found as compared to the spiders (96 vs. 28 records; Tables 1–3) appears to signify this difference in the foraging behavior of the two arachnid groups. Although no experimental studies on harvestmen have been conducted to test whether individuals can survive on an exclusively vegetarian diet, we anticipate that the answer is probably no, because fungi, fruit pulp, seeds, and seed appendages may lack some micro- and macro-nutrients, as well as vitamins, that are exclusively acquired from animal prey (Simpson & Raubenheimer 2012). However, mushrooms, fruit pulp, seeds, and/or seed appendages can make up a substantial portion of the natural diet of harvestmen at times when these food sources are available in high abundance (Machado & Pizo 2000; Schaus et al. 2013; Waldenmaier 2016). Detailed information on the diet of a larger number of species and families will certainly show that consumption of vegetarian food items is widespread in the order Opiliones.
In the case of spiders, fungivory and frugivory appear to be of little relevance for their diet. This might be partially explained by the fact that spiders are “fluid feeders” (Foelix 2011). In fact, spiders feed on vegetarian diets most frequently in the form of fluids, such as nectar, stigmatic exudate, plant sap, and honey dew (e.g., Pollard et al. 1995; Taylor & Foster 1996; Jackson et al. 2001; Nyffeler et al. 2016; Suzuki & Sano 2021). Solid mushroom and fruit tissues are, therefore, not suitable food sources for spiders. An exception occurs when synanthropic spiders feed on fruit waste, because in this particular situation the juice of fruits cut open by human residents can easily be accessed by the spiders. How frequently spiders feed on the liquefied state of the flesh of fruits in natural systems is still unexplored. However, we anticipate that fruits probably do not represent a relevant food item for species of the order Araneae.
ACKNOWLEDGMENTS
North American harvestmen were identified by James C. Cokendolpher (Texas Tech University, USA) and Jeffrey W. Shultz (University of Maryland, USA). Harvestmen from Brazil, Costa Rica, Malaysia, and Singapore were identified by Adriano B. Kury (Museu Nacional do Rio de Janeiro, Brazil), Jochen Martens (University of Mainz, Germany), and Miguel Medrano (Museu Nacional do Rio de Janeiro, Brazil). A harvestman species from Japan was identified by Ryosuke Kuwahara (Kumamoto University Graduate School, Japan). A Neotropical araneid spider was identified by Antonio D. Brescovit (Instituto Butantan, Brazil) and an Australian sparassid by Robert Raven (Queensland Museum, Australia). North American salticids were identified by G.B. Edwards (Florida State Collection of Arthropods, Gainesville, USA) and David Hill (Peckham Society, USA). North American mushrooms of the genus Russula were identified by Tim Baroni (State University of New York, Cortland, USA), Bart Buyck (Muséum National d'Histoire Naturelle, France), and Kathie Therese Hodge (Cornell University, USA). Mushrooms in the families Marasmiaceae and Mycenaceae from Malaysia and Singapore were identified by Dennis E. Desjardin (San Francisco State University, USA) and Serena Lee (Singapore Botanic Gardens, Singapore). Neotropical mushrooms in the families Psathyrellaceae and Pluteaceae were identified by Dennis E. Desjardin (San Francisco State University, USA) and Julieta Carranza (Universidad de Costa Rica, Costa Rica), respectively. Based on a photo, a fungus from Panama was identified by Dennis E. Desjardin, Meike Piepenbring (University of Frankfurt), and Steven L. Stephenson (University of Arkansas, USA) as a slime mold (Myxomycetes). Fruits in the families Combretaceae, Commelinaceae, Fagaceae, Melanthiaceae, Melastomataceae, and Primulaceae were identified by Samantha Koehler and Maria do Carmo do Amaral (both from Universidade Estadual de Campinas, Brazil), Bruce Rottink (formerly Central Research Division, Crown Zellerbach Corp., USA), Renato Goldenberg (Universidade Federal do Paraná, Brazil), and Gustavo Shimizu (Universidade Estadual de Campinas, Brazil), respectively. Kyohsuke Ohkawara (Hokkaido University, Japan) sent us a photo depicting an unspecified harvestman feeding on an elaiosome. We also wish to thank Luis E. Acosta (Universidad Nacional de Córdoba, Argentina) for helping to get access to literature and Norberto Palacios for helping to scan some old slides. Valuable comments of the subject editor Yael Lubin (Ben-Gurion University) and two anonymous reviewers helped to improve the manuscript. Last but not least, we thank the photographers Susannah Anderson (Canada), Gilbert Barrantes (Costa Rica), Nicky Bay (Singapore), Isa S. Betancourt (USA), Rhett Butler (Mongabay, USA), Bruno A. Buzatto (Brazil), Hock Ping Guek (Malaysia), Ryosuke Kuwahara (Japan), Sean McCann (USA), Marco Aurélio Pizo (Brazil), Gustavo S. Requena (Brazil), Andrés Rojas (Costa Rica), Chuck Rosenberger (USA), Bruce Rottink (USA), Martin Suvák (Slovakia), John A. Uribe (Brazil), Rodrigo H. Willemart (Brazil), and Melvyn Yeo (Singapore).
SUPPLEMENTAL MATERIALS
Available online at https://doi.org/10.1636/JoA-S-22-015.s1
Appendix 1 (01. JoA 22-015 Supplemental Material.pdf).—Combinations of key words used in the literature search in all databases.
Figure S1 (01. JoA 22-015 Supplemental Material.pdf).— Further cases of fruit consumption by harvestmen.